Embryonic Stem Cell Extracellular Vesicles Show New Potential to Slow Cellular Aging
Researchers from the Cornell University College of Veterinary Medicine (CVM) have reported a major advance in aging biology, identifying a precise molecular mechanism through which embryonic stem cells can protect other cells from aging. Their findings, published in the Journal of Biological Chemistry in 2025, focus on tiny biological structures known as extracellular vesicles and how these vesicles can prevent cells from entering senescence, one of the core hallmarks of aging.
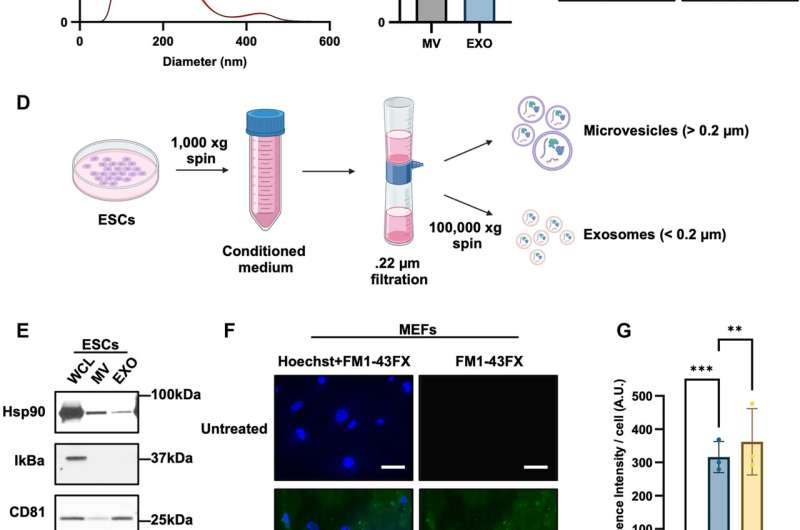
At its core, the study explains how embryonic stem cells release large quantities of microscopic membrane-bound particles—specifically microvesicles and exosomes—that actively communicate with neighboring cells. When these vesicles interact with differentiated, non-stem cells, they can dramatically alter how those cells respond to stress and aging-related damage.
How Embryonic Stem Cells Protect Other Cells
Embryonic stem cells (ESCs) are known for their ability to divide indefinitely and develop into virtually any cell type in the body. This study reveals that part of this “youthful” power lies not just within the cells themselves, but in what they secrete.
The researchers isolated extracellular vesicles derived from mouse embryonic stem cells and introduced them into laboratory dishes containing differentiated fibroblast cells. Fibroblasts are a standard model for studying aging because they reliably enter cellular senescence after repeated division or exposure to stress.
The results were striking. Cells treated with embryonic stem cell vesicles continued dividing and functioning normally, while untreated cells stopped growing and developed classic signs of senescence. These signs include halted cell division, altered morphology, and impaired cellular function.
In simple terms, the vesicles appeared to shield aging cells from the biological signals that tell them to stop working.
Understanding Cellular Senescence and Oxidative Stress
Cellular senescence is a state where cells permanently stop dividing but do not die. While senescence plays useful roles in development and cancer prevention, its accumulation over time contributes to aging, inflammation, and tissue dysfunction.
One of the main drivers of senescence is oxidative stress. This occurs when cells accumulate excessive reactive oxygen species (ROS), which damage proteins, DNA, and cell membranes. Over time, oxidative stress pushes cells into a senescent state.
The Cornell researchers focused on whether embryonic stem cell vesicles could interfere with this process. Their experiments confirmed that the vesicles actively block oxidative stress signaling, preventing the molecular cascade that normally forces cells into senescence.
The Key Molecular Pathway Behind the Anti-Aging Effect
A major strength of this study is that it does not stop at observation—it carefully maps out the protein signaling pathway responsible for the anti-aging effect.
The process begins with fibronectin, an extracellular matrix protein found coating the surface of the embryonic stem cell vesicles. Fibronectin plays a crucial role in cell adhesion and communication, and in this case, it acts as the entry point for the vesicles’ protective effects.
When fibronectin on the vesicle surface binds to receptors on target cells, it activates a sequence of intracellular enzymes, including FAK (Focal Adhesion Kinase) and AKT, both of which are well-known regulators of cell survival and stress resistance.
Activation of the FAK–AKT pathway leads to:
- Suppression of oxidative stress responses
- Increased cellular resilience
- Prevention of senescence-related signaling
By identifying this exact pathway, the study provides a clear biochemical explanation for how embryonic stem cells exert anti-aging effects on surrounding cells.
Why This Discovery Matters for Aging Research
This work represents an important shift in how scientists think about aging interventions. Instead of replacing aged cells or directly modifying DNA, the findings suggest that cell-free therapies, based on extracellular vesicles, could potentially slow or prevent aging-related decline.
Extracellular vesicles are especially attractive from a therapeutic perspective because:
- They are naturally occurring biological messengers
- They can be isolated and purified
- They avoid many ethical and safety issues associated with whole stem cell transplantation
By pinpointing fibronectin and the FAK–AKT signaling axis, the study also opens the door to targeted drug development. In theory, therapies could be designed to mimic or enhance this pathway without requiring stem cells themselves.
Next Steps: From Cells to Living Organisms
While the current research was conducted in cell cultures, the team has made it clear that this is only the beginning. The next phase of research involves testing embryonic stem cell-derived vesicles in mice to determine whether the same anti-aging effects occur at the level of entire organisms.
If successful, future studies will explore similar approaches using human cells, with an important caveat. Any human embryonic stem cells used would not come from embryos but would instead be created by genetically reprogramming adult cells into an embryonic-like state, commonly known as induced pluripotent stem cells (iPSCs).
This distinction is critical both ethically and scientifically, as it allows researchers to study embryonic-like properties without relying on traditional embryonic sources.
Extracellular Vesicles and Their Broader Role in Biology
Extracellular vesicles have become one of the most exciting areas of modern cell biology. Once dismissed as cellular debris, they are now recognized as powerful communication tools that transport proteins, lipids, and genetic material between cells.
Beyond aging, extracellular vesicles are already being studied for roles in:
- Wound healing and tissue regeneration
- Immune system regulation
- Cancer progression and suppression
- Neurodegenerative diseases
This study adds aging prevention to that growing list and reinforces the idea that vesicles can profoundly reshape cellular behavior without altering a cell’s genetic code.
Implications for Human Health and Longevity
Importantly, the researchers emphasize that the goal is not simply extending lifespan. Instead, the focus is on healthspan, the length of time a person remains healthy, functional, and free from chronic disease.
By preventing cellular senescence and oxidative damage, therapies based on extracellular vesicles could one day help:
- Maintain tissue function with age
- Reduce chronic inflammation
- Delay age-related diseases
While clinical applications are still far off, this research provides a solid mechanistic foundation for future anti-aging strategies.
Final Thoughts
This study offers a rare combination of compelling results and molecular clarity. By demonstrating how embryonic stem cell-derived extracellular vesicles delay cellular aging—and by identifying the exact signaling pathway involved—the research marks a significant step forward in understanding how aging might be slowed at the cellular level.
As further animal and human studies unfold, extracellular vesicles may emerge as one of the most promising tools in the ongoing effort to preserve health and vitality over time.
Research Paper:
https://doi.org/10.1016/j.jbc.2025.110821





