Essential Proteins in E. coli Are More Likely to Be Repaired After Misfolding, According to New Research
Proteins are the workhorses of every living cell, but before they can do their jobs, they must fold into precise three-dimensional shapes. When this folding process goes wrong, proteins can lose their function or even become harmful. A new study focused on Escherichia coli (E. coli) has uncovered important details about why certain proteins misfold more often and why some of them are repaired more efficiently than others. The findings highlight a previously underappreciated protein-folding mechanism and suggest that evolution has fine-tuned repair systems to prioritize life-essential proteins.
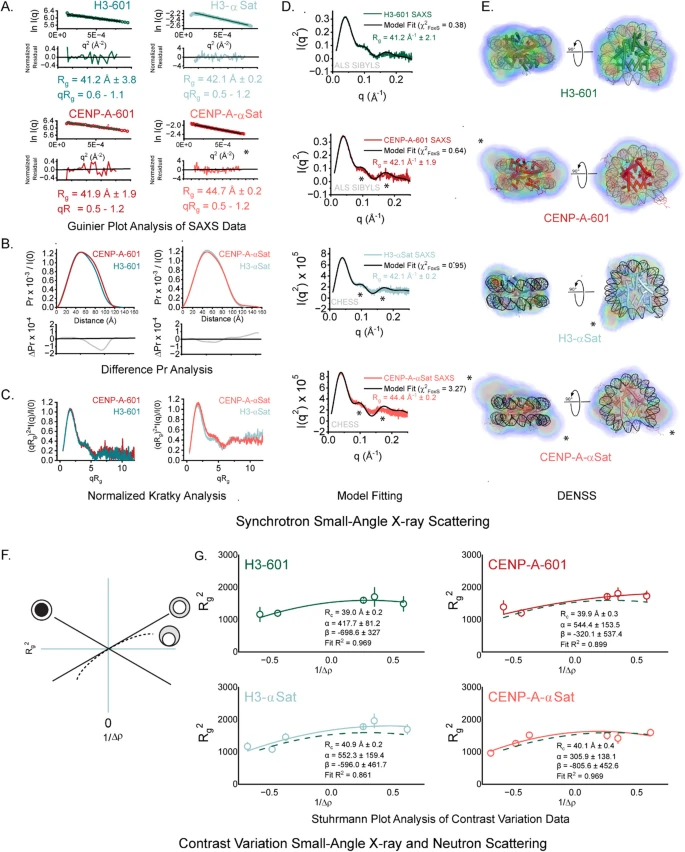
The research, conducted by scientists at Penn State and published in Nature Communications in 2025, centers on a structural feature called a non-covalent lasso entanglement, or NCLE. This motif appears in the native structures of many proteins and plays a surprisingly large role in misfolding and repair.
What Is a Non-Covalent Lasso Entanglement?
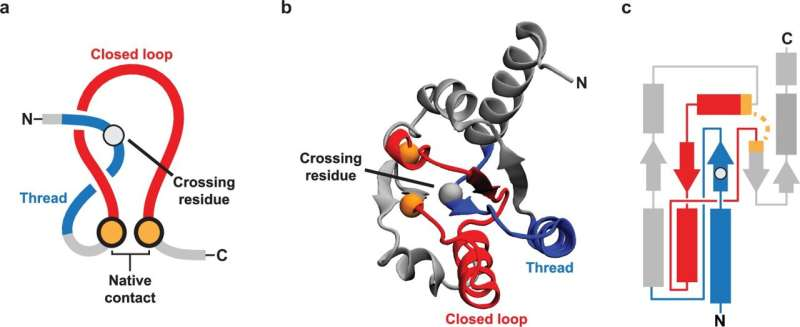
Proteins are long chains of amino acids that fold into loops, helices, sheets, and complex 3D shapes. An NCLE forms when a loop in the protein chain closes via a non-covalent contact, and another segment of the protein threads through that loop, creating a lasso-like entanglement. The contact that closes the loop is not a permanent chemical bond, but a weaker interaction between amino acids that are close together in space.
This structure is not rare. NCLEs are found across many proteins and are part of their normal, functional shapes. However, the same feature that makes NCLEs useful also makes them risky. During folding, timing is critical. If the loop closes before the correct segment threads through it, or if the loop captures the wrong segment, the protein can become trapped in a misfolded state. NCLEs can also form where they do not belong, further increasing the chance of error.
A New and Widespread Misfolding Mechanism
Until recently, scientists knew only a limited number of ways proteins could misfold. This study builds on earlier work that identified loss or misformation of NCLEs as a distinct misfolding mechanism. The Penn State researchers wanted to know how common this mechanism is and whether cells are capable of fixing these mistakes.
To answer this, they analyzed preexisting, publicly available proteomics data from an earlier large-scale study of the E. coli proteome. That dataset compared two nearly identical samples of all E. coli proteins. In one sample, proteins were chemically denatured and then allowed to refold, increasing the likelihood of misfolding. In the other sample, proteins were kept in their native, undisturbed state.
Both samples were then treated with enzymes that cut proteins into smaller fragments. Because enzymes can only cut regions they can physically access, the pattern of fragments reveals information about a protein’s three-dimensional structure. Misfolded proteins expose different regions than properly folded ones, leading to distinct cutting patterns that can be detected using mass spectrometry.
Proteins With NCLEs Misfold More Often
When the researchers compared proteins with and without NCLEs, the difference was striking. Proteins that contain NCLEs in their native structures were found to be about twice as likely to misfold during refolding experiments. Even more notably, misfolding events were around 40% more likely to occur specifically at the NCLE site rather than elsewhere in the protein.
This result suggests that NCLEs represent a structural weak point in the folding process. While they are stable once formed correctly, they are also vulnerable during folding, when small timing errors can lead to long-lived misfolded states.
Chaperones Step In, but Not Equally for All Proteins
Cells are not defenseless against misfolding. They rely on molecular chaperones, specialized proteins that help other proteins fold correctly or refold after mistakes. The researchers examined additional datasets in which denatured E. coli proteins were allowed to refold in the presence of chaperones.
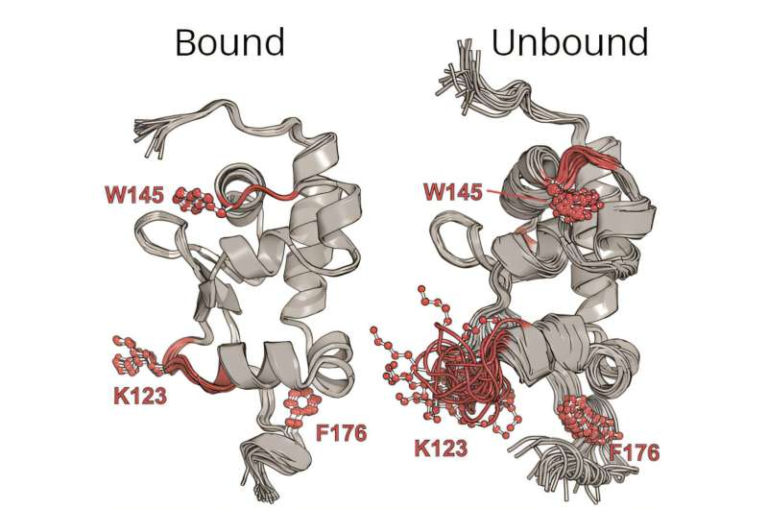
This is where the story takes an especially interesting turn. The team discovered that protein essentiality matters. Proteins that are essential for bacterial survival were rescued by chaperones at a much higher rate than non-essential proteins. In other words, when essential proteins misfold at NCLE sites, the cell’s quality-control machinery is more likely to fix them.
Non-essential proteins, by contrast, were less likely to be rescued. Their misfolded NCLEs often persisted, suggesting that the cell tolerates a higher level of damage when survival is not immediately threatened.
Evolutionary Clues Hidden in Protein Structure
Digging deeper, the researchers analyzed the amino acids responsible for closing NCLE loops. They found a statistically significant difference between essential and non-essential proteins. In essential proteins, the loop-closing interactions tended to be weaker. In non-essential proteins, these same interactions were generally stronger.
This structural difference may be an evolutionary adaptation. Weaker loop closures could allow chaperones easier access to misfolded NCLEs, making repair more efficient. For proteins that are critical to life, natural selection may have favored structures that are not only functional, but also repairable.
Non-essential proteins, on the other hand, would face less evolutionary pressure to remain easily fixable. If they misfold and remain dysfunctional, the consequences for the cell are less severe.
Why Reusing Data Matters
One notable aspect of this study is its approach. Rather than generating entirely new experimental data, the researchers reused and reanalyzed existing datasets. By combining structural databases with large-scale proteomics results, they were able to uncover new insights without repeating costly experiments.
This kind of data reuse is increasingly important in modern biology, where massive datasets already exist but often hold untapped information. The study demonstrates how combining computational analysis with experimental data can reveal patterns that are invisible at smaller scales.
Why This Research Matters Beyond Bacteria
Although this work focused on E. coli, its implications reach far beyond bacteria. NCLE-type misfolding occurs in many organisms, including humans. Protein misfolding is linked to numerous diseases, particularly neurodegenerative disorders where faulty proteins accumulate and damage cells.
Understanding how certain misfolded structures escape or receive repair could help scientists better understand why some proteins are more dangerous when they misfold, and why cells sometimes fail to correct these errors. While this research does not directly address human disease, it adds an important piece to the broader puzzle of protein quality control.
Looking Ahead
This study represents the first high-throughput analysis of NCLE misfolding across hundreds of proteins. It shows that misfolding is not random, that certain structural motifs are especially vulnerable, and that cells actively prioritize the repair of proteins that matter most for survival.
Future research may explore whether similar repair biases exist in more complex organisms and whether these principles can be harnessed in biotechnology or medicine. For now, the findings offer a clearer view of how structure, function, and evolution intersect at the molecular level.
Research paper:
https://www.nature.com/articles/s41467-025-66236-3