Genetically Modified Phages Deliver Bacterial Toxins to Defeat Multidrug-Resistant E. coli
In a promising step toward tackling the growing menace of antibiotic-resistant bacteria, researchers at the Vrije Universiteit Brussel (VUB) and the Flanders Institute for Biotechnology (VIB) have developed a groundbreaking phage-based therapy. The study, led by biologist Jessie Vandierendonck and published in Microbiology Spectrum, introduces a new way to fight multidrug-resistant Escherichia coli using genetically engineered bacteriophages—viruses that infect bacteria.
This new approach doesn’t just rely on the natural bacterial-killing ability of phages. Instead, it combines phage therapy with bacterial toxin–antitoxin systems, offering a method that targets harmful bacteria with precision while sparing beneficial microbes.
The Growing Crisis of Antibiotic Resistance
Antibiotic resistance has become one of the most pressing global health threats. Decades of overuse and misuse of antibiotics in medicine and agriculture have led many bacterial strains to evolve defense mechanisms, rendering standard drugs ineffective.
The World Health Organization (WHO) has listed Escherichia coli among the priority pathogens for which new treatments are urgently needed. While E. coli is a normal resident of the human gut and mostly harmless, certain strains—especially enterohemorrhagic and uropathogenic varieties—can cause severe intestinal infections, urinary tract infections, and even life-threatening sepsis.
As antibiotics lose their edge, scientists have begun revisiting an older but potent concept: phage therapy.
How Bacteriophages Work
A bacteriophage (or simply phage) is a virus that infects bacteria. Imagine a tiny lunar lander attaching itself to a bacterial cell—this is how a phage binds to bacterial receptors on the cell wall. Once attached, the phage injects its genetic material into the host, hijacking the bacterium’s cellular machinery to produce more phages.
There are two main routes a phage can take:
- The lytic cycle, where the phage replicates inside the host until it bursts the cell open, killing it in the process.
- The lysogenic (or temperate) cycle, where the phage integrates its genetic material into the bacterial genome, lying dormant until certain conditions activate it.
Most phage therapies use lytic phages because they directly destroy bacteria. However, temperate phages—those that can integrate into bacterial DNA—have unique advantages when engineered properly. This is exactly where Vandierendonck’s research breaks new ground.
The Innovation: Phages That Deliver Toxins
Vandierendonck’s team developed a novel phage-based system that uses temperate phages as vehicles to deliver bacterial toxin genes directly into harmful E. coli cells. These toxins, derived from natural toxin–antitoxin systems found in bacteria, are lethal when expressed inside the bacterial cell.
The concept is elegantly simple yet technically demanding:
- The phage infects the target bacterium.
- Once inside, it delivers the genetic blueprint for a toxin.
- The bacterium itself produces the toxin, which kills it from within.
This method has two major advantages. First, it allows for high precision, ensuring only the harmful bacteria are affected. Second, it reduces collateral damage to beneficial gut bacteria that are often wiped out by conventional antibiotics.
Overcoming Genetic Engineering Challenges
Engineering toxin-bearing phages isn’t easy. The biggest challenge lies in cloning toxin genes—these genes can kill the very bacterial cells used to produce the phages. To solve this, Vandierendonck’s team devised new cloning strategies that tightly regulate toxin gene expression at multiple control points. This ensured that the bacterial host cells stayed alive long enough for the phages to be assembled and harvested.
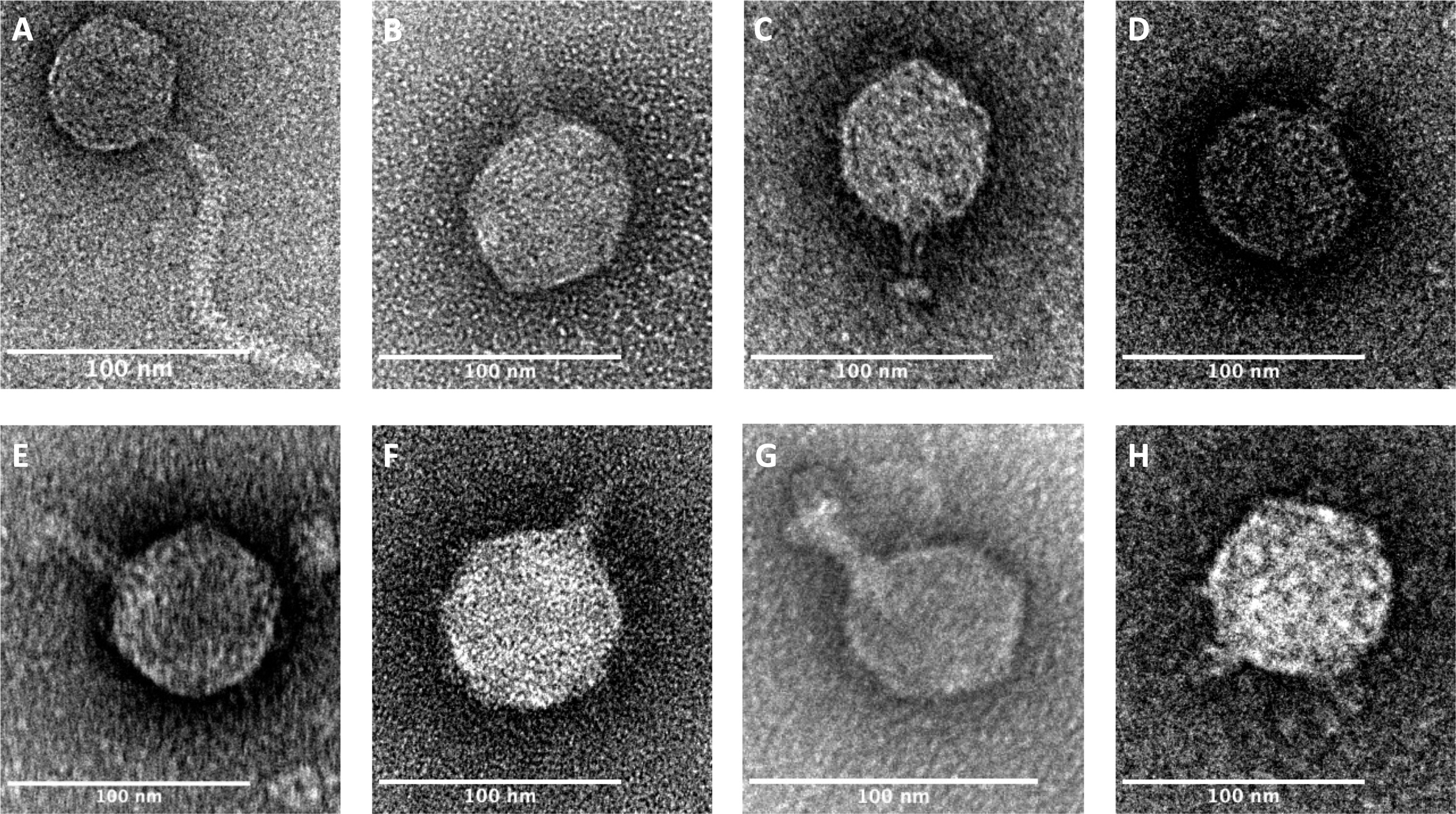
In her experiments, Vandierendonck isolated several phages from enterohemorrhagic E. coli strains and characterized them both genetically and morphologically. Advanced negative-staining electron microscopy was used to capture detailed images of the phage structures, showing their diversity and confirming their suitability for the engineered approach.
Each phage—labeled with identifiers like phi330, phi296, phi315, phi345, phi346, phi349, phi367, and phi419—was studied to determine its shape, genetic composition, and the bacterial receptors it recognizes. These details were crucial for selecting the right candidate phage for toxin delivery.
As a proof of concept, one of the characterized phages was successfully combined with toxin genes. When tested in the lab, the engineered phage effectively killed its target bacterium, validating the approach.
Why This Matters
This innovation represents more than just a laboratory success—it’s a conceptual leap in how we might fight bacterial infections in the future. By harnessing phages not just as bacterial killers but as smart delivery systems, scientists can design precision tools that act only where needed.
Unlike broad-spectrum antibiotics, which often upset the microbiome by killing beneficial bacteria, phage-based treatments can target specific strains. This could mean fewer side effects, reduced antibiotic resistance pressure, and more sustainable infection management.
If developed further, such engineered phages could play a critical role in treating infections that no longer respond to antibiotics, including those caused by multidrug-resistant E. coli, Klebsiella, and Pseudomonas.
A Look Into the Future of Phage Therapy
While the proof-of-concept results are encouraging, several challenges remain before this technology reaches clinical application:
- Safety testing: Researchers must ensure the toxins are expressed only in targeted bacteria and that engineered phages don’t transfer genes to unintended hosts.
- Host range: Phages typically have a narrow range of bacteria they can infect. Expanding this range while maintaining precision will be key.
- Resistance risk: Just as bacteria can become resistant to antibiotics, they can evolve mechanisms to evade phages.
- Regulatory hurdles: Using genetically modified viruses in medicine raises complex safety and ethical questions that will require new regulatory frameworks.
- Manufacturing: Large-scale, consistent production of customized phages will be necessary for widespread clinical use.
Despite these hurdles, the field of synthetic biology is rapidly advancing, offering tools to overcome many of these limitations. Researchers are now exploring hybrid phages that can deliver CRISPR systems, enzymes, or gene silencers to disable bacterial virulence or resistance mechanisms—expanding far beyond toxin delivery.
About the Researcher
Jessie Vandierendonck, who led this study, completed her Master’s in Biology at VUB in 2020 before starting her Ph.D. within the Structural Biology Brussels research group under Professor Remy Loris. During her doctoral studies, she published multiple first-author papers, mentored Master’s students, and presented at several international bacteriophage conferences.
Her work reflects a broader scientific effort to create targeted, sustainable, and safe antimicrobial treatments that can one day replace or complement traditional antibiotics.
What This Means for the Fight Against Superbugs
Antimicrobial resistance (AMR) is projected to cause 10 million deaths per year by 2050 if left unchecked. Conventional antibiotics alone cannot reverse this trend. Phage therapy, especially in its engineered form, represents a renewed hope in this battle.
If therapies like Vandierendonck’s move successfully from laboratory testing to clinical trials, we could be entering a new era of precision antimicrobials—custom-built biological tools that eliminate pathogens with surgical accuracy.
This shift wouldn’t just change how infections are treated. It would transform how we think about medicine, shifting the focus from chemical drugs to biological and genetic therapies designed for the microbial world’s complexities.