Glowing Amino Acid Sensors Reveal the Hidden Dance of Proteins Inside Living Cells
Scientists at Rice University have developed an extraordinary way to watch what’s happening inside living cells — in real time. Their new system uses a 21st amino acid that glows when proteins undergo tiny molecular changes, helping researchers observe the processes that drive everything from growth and aging to cancer development. The study, published in Nature Communications (2025), introduces a tool that could transform how we study biology at the molecular level — without damaging or disrupting cells.
A New Way to See Inside Living Cells
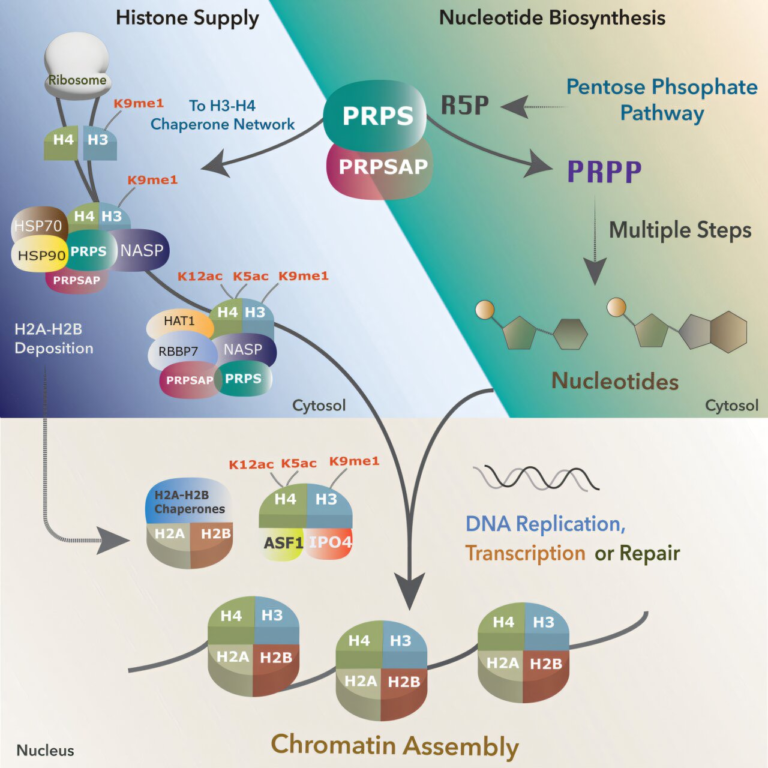
Proteins are the engines of life. They constantly change their structure and behavior through small chemical tweaks known as post-translational modifications (PTMs). These PTMs act like biological on/off switches, controlling cell growth, repair, communication, and even the onset of diseases.
Traditionally, studying PTMs has been a painful process. Scientists have had to break open cells, use chemical dyes, or rely on synthetic labels to catch a glimpse of these modifications. Those methods work, but they can distort what’s actually happening in living systems.
That’s where this new Rice University breakthrough comes in. The researchers engineered cells to produce a glowing version of the amino acid lysine, known as acetyllysine, completely inside the cell — no external chemicals required. When a PTM occurs, the glow changes, creating a real-time light signal that shows how proteins behave inside living organisms.
This is a massive leap forward because it gives scientists the ability to observe protein regulation naturally, inside living cells, without interfering.
How It Works: Building a 21st Amino Acid
Every living organism uses 20 standard amino acids to build proteins. The Rice team decided that wasn’t enough. They wanted to add a new one — the 21st amino acid — that could act as a live reporter of cellular changes.
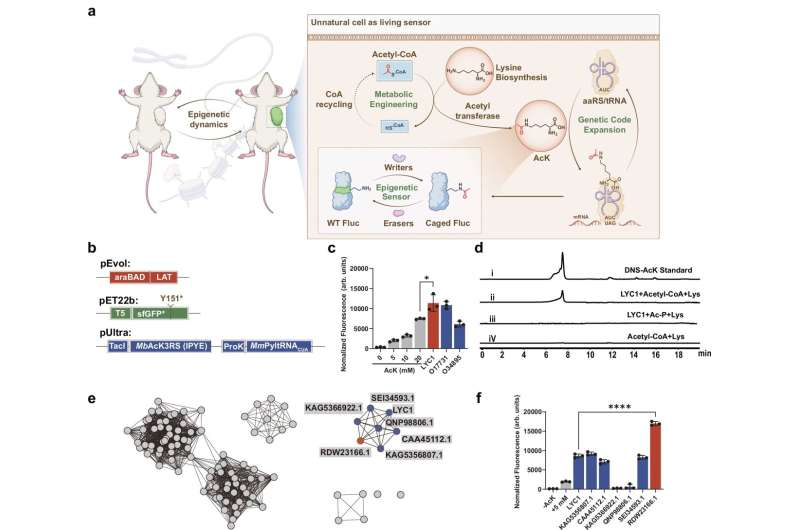
They achieved this by designing cells to produce acetyllysine (AcK) on their own. Normally, researchers would feed cells synthetic AcK, which is both inefficient and disruptive. Instead, the Rice team identified enzymes capable of producing AcK directly inside the cell by combining lysine (a common amino acid) with acetyl-CoA, a key metabolic molecule.
To get this new amino acid into proteins, they used a genetic code expansion technique. Essentially, they reprogrammed the cells’ genetic machinery to insert acetyllysine at specific positions in a protein’s structure. A special tRNA/synthetase pair recognizes an unused stop codon (called the TAG codon) and places AcK there during protein synthesis.
Once incorporated, these proteins could emit light — glowing differently when certain modifications occurred. This allowed scientists to literally watch proteins change state inside living bacteria, human cells, and even tumor models.
Testing the Sensors in Living Systems
The team tested the glowing sensors in bacteria, human cells (HEK293T and HCT116), and animal tumor models. Using fluorescent and bioluminescent reporter proteins, they proved that acetyllysine could be successfully made and inserted into proteins inside living cells.
When post-translational modifications occurred — for instance, when enzymes added or removed acetyl groups — the glow changed. This provided instant feedback about what was happening at the molecular level.
For verification, they used liquid chromatography–mass spectrometry (LC–MS) to confirm that the cells were indeed synthesizing and incorporating acetyllysine correctly.
The researchers then focused on one specific enzyme: SIRT1, a well-known deacetylase that helps regulate inflammation, aging, and cancer biology. They found that blocking SIRT1’s enzymatic activity affected protein acetylation, but, interestingly, did not stop tumor growth in some cell lines. This finding challenges long-held assumptions about how SIRT1 functions in cancer — showing that biological systems can behave in unexpected ways when observed in real time.
Why This Matters
This technology opens up entirely new possibilities for biology. Being able to track PTMs as they happen means researchers can study cellular processes without breaking the system they’re studying.
In simpler terms, it’s like watching a play unfold live on stage, instead of trying to guess the story from a pile of broken props afterward.
Because this method works in living organisms — not just in test tubes — it offers powerful tools for studying cancer, aging, and neurological diseases. It could also accelerate drug discovery, as scientists will be able to screen compounds that influence PTM enzymes more quickly and accurately.
The Rice team believes this system could soon be expanded to monitor other types of modifications, like methylation or phosphorylation, and even applied in human organoid models. That would bring us closer to personalized medicine, where therapies are guided by real-time cellular behavior.
The Science Behind Post-Translational Modifications
To appreciate the full impact of this research, it’s worth understanding what PTMs actually are.
After a protein is made, it doesn’t just go to work immediately. It often gets chemically modified — for example, an enzyme might attach a small group like an acetyl, phosphate, or methyl group to it. These modifications can:
- Change how a protein folds or interacts with others.
- Turn a protein’s activity on or off.
- Determine where it goes inside the cell.
- Affect how long it survives before being degraded.
PTMs are central to how cells adapt to stress, regulate metabolism, and defend against disease. But because they happen so quickly and subtly, seeing them in living systems has been nearly impossible — until now.
The Rice team’s glowing sensors make these invisible processes visible, potentially leading to insights into how epigenetic regulation shapes health and disease.
Beyond Cancer: Broader Applications
While the initial tests focused on cancer-related enzymes, the implications are much broader. Many neurological and metabolic diseases — like Alzheimer’s, Parkinson’s, and diabetes — involve abnormal PTM activity. If scientists can track these changes in living tissues, they might identify new drug targets or better understand how these diseases progress.
The approach could also help researchers study aging, since acetylation and other PTMs play key roles in lifespan regulation. By following how proteins change over time in living cells, scientists could uncover the molecular switches that slow or accelerate aging.
Moreover, because the glowing signals are light-based, this system could be adapted for high-throughput drug screening. Automated imaging systems could watch thousands of glowing cells at once, rapidly identifying compounds that modulate specific PTM enzymes.
Challenges and Next Steps
As with any new technology, there are hurdles ahead. The researchers note that tissue heterogeneity, oxygen levels, and nutrient conditions can influence how the sensors behave inside animals. These factors may affect the brightness and accuracy of the glow signals.
There’s also the question of expanding the system to detect other modifications. Each PTM type will require finding or designing a unique biosynthetic pathway and matching tRNA-synthetase pair. That’s complex molecular engineering work, but the success with acetyllysine is a major proof of concept.
Still, the long-term goal is clear: to create a suite of living sensors that can monitor many different biochemical processes inside the body — providing a real-time map of cellular activity.
The Bigger Picture
What Rice University’s team has achieved is both scientifically elegant and profoundly practical. By giving cells the ability to make and use a new amino acid that glows when proteins change, they’ve built a bridge between chemistry, genetics, and live imaging.
This research doesn’t just add a new tool to the molecular biologist’s kit — it changes how we can see life itself at the microscopic level.
The idea that living cells can be programmed to light up their own inner workings feels like something out of science fiction, yet it’s real, functional, and opening up a new frontier in how we study life and disease.
Research Reference:
Engineering Unnatural Cells with a 21st Amino Acid as a Living Epigenetic Sensor – Nature Communications (2025)