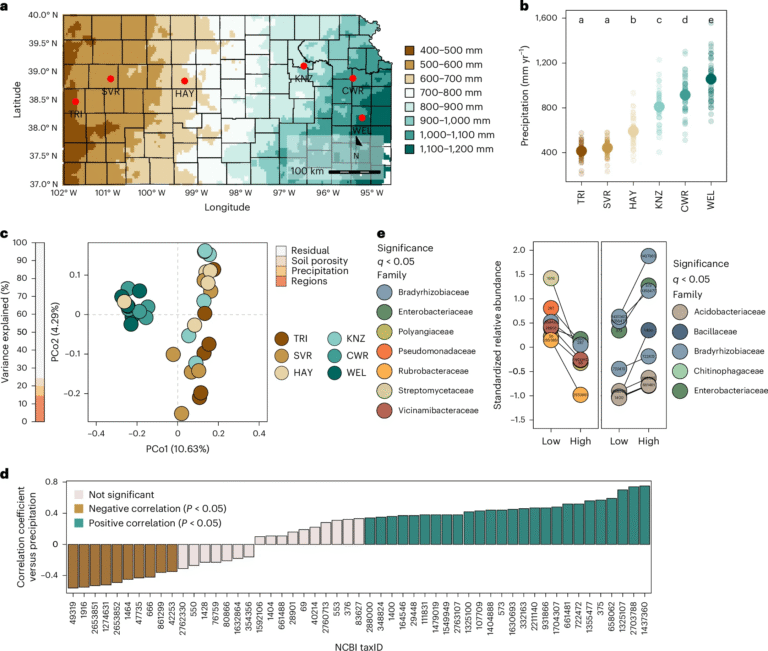
Higher Dose of Semaglutide Shows Nearly 20% Weight Loss in New Clinical Trials

A new pair of large clinical trials has revealed that a higher weekly dose of semaglutide (7.2 mg) can help adults with obesity lose significantly more weight compared to the currently approved 2.4 mg dose or a placebo. These results were published in The Lancet Diabetes & Endocrinology and mark the first time researchers have tested whether raising the semaglutide dose beyond the approved level could bring greater benefits.
The findings come from two separate phase 3b trials: one involving adults with obesity but without diabetes (called STEP UP), and another involving adults with both obesity and type 2 diabetes (called STEP UP T2D). Both trials lasted 72 weeks and included lifestyle support such as dietary guidance and physical activity recommendations, ensuring participants were not only relying on the drug alone.
Trial Design and Participants
The STEP UP trial enrolled about 1,407 adults with obesity but no diabetes. Participants were randomly assigned in a 5:1:1 ratio to either receive semaglutide 7.2 mg, semaglutide 2.4 mg, or a placebo once a week.
The STEP UP T2D trial enrolled around 512 adults with obesity and type 2 diabetes across 68 sites in 8 countries. Here, 307 participants received 7.2 mg, 103 received 2.4 mg, and 102 received placebo.
At the start of the studies, participants had a mean BMI of around 39.8 kg/m² and an average weight of about 112.4 kg in the 7.2 mg groups. All participants also received structured dietary guidance (around a 500-calorie daily reduction) and advice to engage in at least 150 minutes of physical activity per week.
Results in Adults Without Diabetes (STEP UP)
In people without diabetes, the results were striking.
- On average, those who took 7.2 mg semaglutide lost nearly 20.7% of their body weight, compared with 17.5% for those on 2.4 mg, and only 2.4% for placebo.
- Using the treatment policy approach (which accounts for dropouts and adherence), the average reduction for 7.2 mg was about –18.2% compared to placebo and about –3.2% greater than 2.4 mg.
- Importantly, 50.9% of people on the higher dose lost at least 20% of their starting body weight. For comparison, 16.7% achieved this on 2.4 mg, and just 2.9% on placebo.
- A substantial 33.2% of the 7.2 mg group achieved 25% or more weight loss, compared to virtually none in the placebo group.
- Body Mass Index (BMI) outcomes were also notable. In the 7.2 mg group:
- 43.2% dropped below a BMI of 30 (the obesity cutoff).
- 36% reached a BMI under 27.
- 15.6% achieved a BMI under 25 (the “normal” range).
In addition to overall weight reduction, participants also saw improvements in waist circumference and waist-to-height ratio. The higher dose group reduced their waist circumference by an estimated 11.7 cm more than placebo, and about 2.9 cm more than the 2.4 mg group. Around 25.4% of participants achieved a waist-to-height ratio below 0.53, compared to just 4.7% on placebo.
Results in Adults With Type 2 Diabetes (STEP UP T2D)
Weight loss is often harder for people with type 2 diabetes, but even here the 7.2 mg dose delivered significant results.
- The average weight loss was –13.2% with 7.2 mg, compared to –10.4% with 2.4 mg and –3.9% with placebo.
- More patients on 7.2 mg reached clinically meaningful weight-loss targets such as ≥20% reduction in body weight.
- Improvements were also observed in blood sugar (HbA1c levels), waist size, and other obesity-related risk factors.
Safety and Side Effects
The big question with higher doses is whether the safety profile changes.
- Across both studies, semaglutide 7.2 mg was generally safe and well tolerated. There was no increase in serious adverse events or severe hypoglycemia compared to the standard dose or placebo.
- The most common side effects were gastrointestinal — nausea, diarrhea, and vomiting. These were usually most noticeable during the dose-escalation period and tended to settle down over time.
- A less common but more dose-related side effect was dysaesthesia (a tingling or altered touch sensation). About 20% of patients on the 7.2 mg dose reported this, compared with around 5% in the 2.4 mg group.
- Most side effects were manageable and did not lead to participants dropping out of the study.
One point raised by experts is the need to monitor how much of the weight lost is fat mass versus lean muscle mass. With such large amounts of weight loss, preserving muscle becomes an important consideration.
Expert Perspectives
Experts welcomed the findings but added important caveats:
- The effectiveness of 7.2 mg is clear, with nearly half of participants without diabetes losing at least 20% of their body weight.
- However, the trials were not designed to fully compare 7.2 mg against 2.4 mg in every subgroup. The main goal was to establish superiority over placebo.
- Some specialists noted that while improvements versus placebo were dramatic, the extra benefit over 2.4 mg—particularly in people with diabetes—was modest.
- Questions remain about the long-term safety profile, especially since these trials only lasted 72 weeks. Regulators will want to see more data on heart, kidney, and other organ health outcomes.
Why This Matters
Obesity is one of the leading global health challenges, linked with type 2 diabetes, heart disease, certain cancers, and reduced life expectancy. Even a 5–10% weight loss can improve health outcomes, and here we’re talking about average reductions of 13–20% depending on the group.
The currently approved 2.4 mg dose of semaglutide (marketed as Wegovy for obesity and Ozempic for diabetes) has already been considered one of the most effective medical weight loss tools. But for many people, progress plateaus before reaching their health goals. The 7.2 mg dose could provide an additional option for those who need more help.
What Is Semaglutide?
Semaglutide is a GLP-1 receptor agonist, a type of medication originally developed for type 2 diabetes. It mimics a hormone that helps regulate blood sugar, appetite, and digestion.
At lower doses (like 1 mg), it is sold as Ozempic for type 2 diabetes. At 2.4 mg, it is approved as Wegovy for chronic weight management in people with obesity or overweight with weight-related conditions.
The mechanism is straightforward: semaglutide helps people feel full sooner and stay full longer, reduces cravings, and improves blood sugar control. Combined with lifestyle changes, it has proven to be one of the most effective weight-loss drugs available today.
Broader Context: Other Drugs in the Same Space
Semaglutide is part of a rapidly growing field of anti-obesity medications. Another drug, tirzepatide (sold as Mounjaro for diabetes and Zepbound for obesity), has shown weight loss of over 20% in some studies.
The competition among these drugs is driving pharmaceutical companies to test higher doses, combinations, and newer GLP-1 variants. The question going forward is not just how much weight people can lose, but how sustainable, safe, and accessible these treatments will be in the long run.
What Comes Next
While the results are promising, the 7.2 mg dose is not yet approved. More research will be needed before regulators can consider it for widespread use. Questions about long-term safety, cost, and accessibility will play a big role.
Still, these results highlight just how powerful GLP-1 drugs can be. If approved, the higher dose could be a valuable option for people who have not reached their health goals on the standard dose.