Hitchhiking DNA: How a Hidden Gene Trick Could Save Species from Extinction
Scientists have discovered a fascinating genetic twist that could change how we understand evolution, aging, and the survival of species. A research team led by Hiroki Shibuya at the RIKEN Center for Biosystems Dynamics Research in Japan has found that in the tiny roundworm Caenorhabditis elegans, an essential piece of DNA doesn’t live where scientists expected it to. Instead, it hitchhikes inside another gene — a discovery that may explain how this species keeps its chromosomes intact and avoids extinction.
The study, published in Science in October 2025, reveals that this hidden DNA strategy might be more common in nature than we ever realized. And beyond its biological intrigue, it could also have implications for anti-aging research, fertility treatments, and regenerative medicine in humans.
Understanding Telomeres and Why They Matter
To grasp why this finding is so remarkable, we first need to talk about telomeres. Think of telomeres as the little plastic tips on the ends of shoelaces. They cap and protect the ends of chromosomes, preventing DNA from fraying or getting damaged.
Each time a cell divides, its telomeres get slightly shorter. Over time, this leads to cellular aging — when telomeres become too short, cells either stop dividing or self-destruct. That’s one reason our skin wrinkles, our organs age, and our bodies gradually lose regenerative power.
But not all cells age this way. Germ cells — the cells that produce sperm and eggs — have a special enzyme called telomerase. This enzyme repairs and extends telomeres, ensuring that each new generation starts with fresh, full-length telomeres. Without this, species would lose genetic stability over generations and eventually die out.
The Mystery That Lasted 20 Years
In most organisms, telomerase is made up of a protein component and an RNA template. This RNA acts as the blueprint for adding DNA back onto the telomeres. In humans, the RNA part is produced from a gene called TERC (telomerase RNA component).
Here’s the puzzle: C. elegans clearly has telomerase activity — its telomeres stay intact — but for over two decades, scientists couldn’t find any TERC gene in its genome. The worm seemed to have working telomerase, but no apparent instructions for making the essential RNA component.
Some researchers assumed the TERC gene must have been lost during evolution. Others thought the worm had evolved a completely different mechanism. But none of the theories fully explained how C. elegans maintained its telomeres.
The Breakthrough: An RNA Hidden in Plain Sight
To solve the mystery, Shibuya’s team at RIKEN decided to take a different approach. They genetically engineered C. elegans worms to overproduce the telomerase protein. This allowed them to collect and purify large amounts of the enzyme complex, including any RNA that was attached to it.
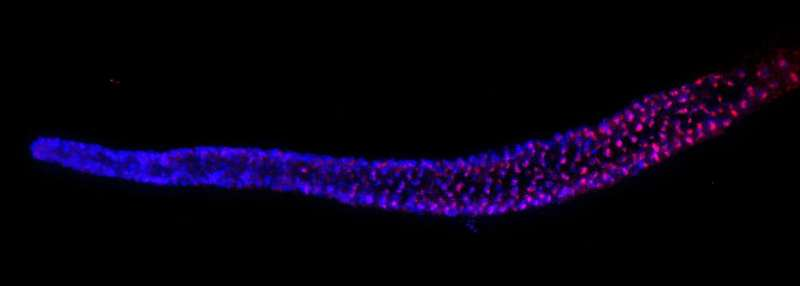
Once they had this RNA, the researchers searched the worm’s genome for matching DNA sequences — and that’s when they found something extraordinary. The RNA wasn’t located in its own gene at all. Instead, it was hidden inside a non-coding region, or intron, of another gene called nmy-2.
Normally, genes are like recipes for making proteins. The parts of a gene that are actually used to make a protein are called exons, while introns are segments that are usually cut out and discarded. But in this case, the intron wasn’t useless at all — it contained a vital RNA that the worm needed to survive.
The team named this hidden RNA terc-1, marking it as the C. elegans equivalent of the missing TERC.
Proving the Hitchhiking Strategy Works
To test whether terc-1 was really essential, the researchers created worms that lacked it. The results were dramatic. Without terc-1, the worms’ telomeres shortened a little more with every generation. By about the 15th generation, the worms became sterile — effectively reaching genetic extinction.
Next, they experimented with putting terc-1 back into different locations in the genome. When they placed it inside introns of other genes that are active in germ cells, the worms’ telomeres stayed normal, and the population survived. But when they placed it inside genes that are only active in somatic cells (the non-reproductive cells in the body), the telomeres still shortened, and the worms eventually died out.
The conclusion was clear: the placement of terc-1 inside germline-active genes ensures that it’s expressed only in the right cells — the ones responsible for passing on genetic material to the next generation. In other words, the terc-1 RNA “hitchhikes” on a germline gene to make sure it’s in the right place at the right time.
What This Means for Evolution and Genetics
This discovery doesn’t just solve a 20-year-old mystery about one worm. It also challenges our broader understanding of how genes and non-coding RNAs can be organized.
The researchers suggest that DNA hitchhiking — where important non-coding RNAs are embedded inside other genes — could be a widespread biological strategy. It may help organisms coordinate gene expression more efficiently, ensuring that critical molecules are produced only when and where they’re needed.
For instance, if the host gene is activated only in germ cells, then the embedded RNA will automatically follow that expression pattern. It’s a clever built-in regulatory system — one that evolution might have used in many species, including possibly our own.
Why Humans Should Care
You might be wondering: why does a worm’s genetic quirk matter to us?
Telomerase and telomeres are deeply connected to human health and aging. When telomeres become too short, cells can no longer divide, contributing to tissue degeneration and aging. On the other hand, too much telomerase activity is linked to cancer, because it lets cells divide indefinitely.
Understanding how telomerase RNA is produced and regulated in different organisms could reveal new ways to fine-tune telomerase activity — helping scientists find safer approaches to delay aging or regenerate tissues without triggering uncontrolled cell growth.
It could also shed light on fertility problems. In humans, telomerase is crucial for the health of eggs and sperm. Subtle defects in how telomerase RNA is made could affect reproductive longevity, just as it does in worms.
So while we’re a long way from “curing aging,” discoveries like this open up fresh ways to study how cells maintain their chromosomes across generations — a key part of what keeps life going.
A Broader Look at DNA Hitchhiking
The idea that RNA or DNA sequences can “hitchhike” inside other genes isn’t entirely new, but this is one of the clearest examples of it playing such a crucial role. Scientists have long known that introns can sometimes host small RNAs, microRNAs, or snoRNAs, but in most cases, these aren’t essential for survival.
The C. elegans terc-1 case is different. It shows that a life-sustaining RNA can be embedded inside another gene and depend on it completely for expression. This adds another layer to our understanding of genomic architecture — suggesting that genes are not as independent as once thought.
As researchers continue to analyze genomes across species, more examples of this hidden regulatory strategy may come to light. The discovery emphasizes that non-coding DNA — often dismissed as “junk DNA” — can play powerful roles in controlling life’s most fundamental processes.
Final Thoughts
The discovery of terc-1 hidden inside the nmy-2 gene in C. elegans is a remarkable reminder that evolution is both creative and efficient. Instead of inventing new genes, sometimes it simply repurposes existing ones.
By embedding essential RNAs inside active genes, nature ensures that critical molecules like telomerase RNA appear exactly when and where they’re needed — protecting the continuity of life itself.
This isn’t just about worms. It’s about how life finds ways to endure, generation after generation, by writing ingenious solutions into its own genetic code.
Research Paper: Nematode telomerase RNA hitchhikes on introns of germline–up-regulated genes — Science (2025)