How a Bacterial Enzyme Uses Vitamin C to Disarm the Immune System
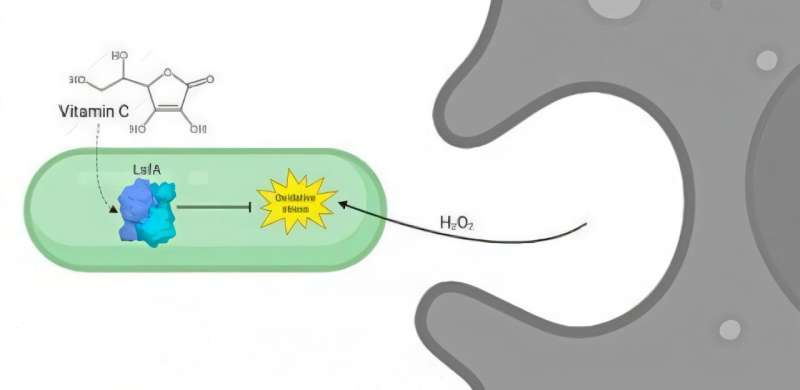
Researchers have uncovered a fascinating new survival trick used by Pseudomonas aeruginosa, a dangerous bacterium known for causing severe hospital-acquired infections. A team led by scientists from the University of São Paulo (IB-USP) and the Center for Redox Processes in Biomedicine (Redoxoma) has discovered that this microbe uses vitamin C to strengthen its defenses against our immune system. Their study reveals the detailed structure and function of a bacterial enzyme called LsfA, showing exactly how it helps the pathogen neutralize one of the immune system’s most powerful weapons: hydrogen peroxide.
This discovery is not just another piece of microbiology trivia—it could open the door to new antibacterial treatments and deepen our understanding of how pathogens evolve to survive the body’s harsh defensive environment. Below is a clear breakdown of the findings, the biological context, and the wider implications.
What the New Study Reveals About LsfA
The star of the study is LsfA, a type 1-Cys peroxiredoxin (often shortened to Prx). Peroxiredoxins are a family of antioxidant enzymes present across many living organisms. Their job is to remove harmful oxidants, especially hydroperoxides, which immune cells use to destroy invading microbes.
In this case, the researchers found that LsfA protects Pseudomonas aeruginosa by breaking down hydrogen peroxide using vitamin C as a reducing agent. This detail is crucial. Previously, scientists believed that this class of peroxiredoxins relied exclusively on thiols—sulfur-containing molecules—to regenerate after oxidation. But this study shows that ascorbate (vitamin C) directly participates in this regeneration process.
The team used structural biology and biochemical analyses to map out the LsfA protein in detail. They obtained the first-ever structural model of a bacterial Prx6 enzyme, something that had never been solved before despite many known structures from Archaea and mammals. Interestingly, even the model bacterium E. coli does not have this type of enzyme, which makes LsfA especially interesting from an evolutionary standpoint.
They also demonstrated that P. aeruginosa contains multiple antioxidant enzymes, but LsfA is exceptionally efficient at decomposing hydrogen peroxide, making it one of the bacterium’s strongest defenses in hostile immune environments.
Why Vitamin C Matters in This Mechanism
One of the most intriguing aspects of the study is the interaction between LsfA and ascorbate. Back in 2007, the same research group showed that vitamin C can reduce a specific oxidized form (sulfenic acid) of 1-Cys peroxiredoxins. But the broader biological significance of this was unclear.
Now, the new structural analyses indicate that ascorbate directly interacts with LsfA’s active site, allowing the enzyme to quickly regenerate after oxidation and continue neutralizing hydrogen peroxide. This reveals a new biological role for vitamin C in bacterial survival.
Researchers used the HyPer7 probe, a tool for tracking hydrogen peroxide inside cells, to observe the process in live Pseudomonas cells. This is the first time the probe has been used in this bacterium, making the results even more significant.
Understanding Pseudomonas aeruginosa and Its Threat Level
To appreciate the importance of this discovery, it helps to understand the bacterium itself. Pseudomonas aeruginosa is an opportunistic pathogen—meaning it primarily targets people with weakened immune systems. It is responsible for serious infections such as:
- Pneumonia in cystic fibrosis patients
- Burn and surgical wound infections
- Urinary tract infections
- Endocarditis
- Septicemia
Because it is highly resistant to antibiotics, the World Health Organization lists it as a priority pathogen for developing new treatments.
When our bodies detect infection, immune cells—especially phagocytes—launch an oxidative attack using reactive oxygen, nitrogen, and chlorine species. This creates intense oxidative stress. To survive, pathogens activate an arsenal of antioxidant systems. One of the most potent among them in P. aeruginosa is the LsfA protein.
What Makes LsfA a Unique Drug Target
An important challenge in designing antibacterial drugs is avoiding harm to human cells. LsfA has a human homolog, meaning humans also have a protein with similar structure and function. However, the researchers found key differences: while LsfA shares broad structural similarities with human Prx6 proteins, it has distinct electrostatic properties at its active site. These charge differences provide a possible path toward designing selective inhibitors that target only the bacterial enzyme.
The team even used in silico docking—computer simulations of molecular interactions—to show how ascorbate binds to LsfA. This kind of information helps scientists imagine what an inhibitor might look like, or how one might block the vitamin C interaction.
Broader Context: How Peroxiredoxins Work
For readers less familiar with these proteins, here’s a quick overview.
Peroxiredoxins (Prxs) are antioxidant enzymes found in bacteria, plants, animals, fungi, and even Archaea. They play major roles in:
- Detoxifying hydrogen peroxide
- Maintaining cellular redox balance
- Regulating signaling pathways
- Protecting cells from oxidative damage
There are multiple types: 1-Cys, 2-Cys, and atypical 2-Cys peroxiredoxins. LsfA belongs to the 1-Cys group, which has long puzzled researchers because its natural reducing agent was not clearly defined across organisms.
This study suggests that in some bacteria, ascorbate may be a key natural reducing partner, expanding our understanding of cellular redox chemistry.
Potential Implications for Future Therapies
If inhibitors can be designed to target LsfA without harming human Prx6, researchers could begin developing a new class of antibacterial drugs. Since P. aeruginosa is notoriously antibiotic-resistant, this represents a promising avenue.
The study authors note several next steps:
- Investigating how P. aeruginosa processes or acquires ascorbate, since its vitamin C metabolism is poorly understood
- Testing LsfA function in macrophage infection models
- Exploring how deletion of the LsfA gene affects immune response and inflammation
- Studying additional differences between bacterial and human Prx6 forms to improve inhibitor design
Given how destructive P. aeruginosa infections can be, any advance in understanding its survival mechanisms has meaningful therapeutic potential.
Additional Background on Hydrogen Peroxide and Immune Defense
Hydrogen peroxide (H₂O₂) is not just a cleaning agent in your medicine cabinet—it is a key molecule used by the immune system. Immune cells release it to damage or kill pathogens. Many bacteria have evolved specialized enzymes to neutralize this threat, including:
- Catalases
- Peroxidases
- Peroxiredoxins
What makes the LsfA discovery important is that it reveals a previously unknown strategy for bypassing this immune attack.
A Quick Look at Vitamin C’s Chemical Role
Vitamin C (ascorbate) is widely known as an antioxidant in humans, protecting cells from oxidative damage. Its properties include:
- Donating electrons
- Reducing oxidized molecules
- Regenerating certain enzymes
- Supporting immune function
This study shows that pathogens can exploit vitamin C’s reducing power to protect themselves, which adds an interesting twist to how we typically think about antioxidants.
Research Paper
Interaction between 1-Cys peroxiredoxin and ascorbate in the response to H2O2 exposure in Pseudomonas aeruginosa
https://doi.org/10.1016/j.redox.2025.103658