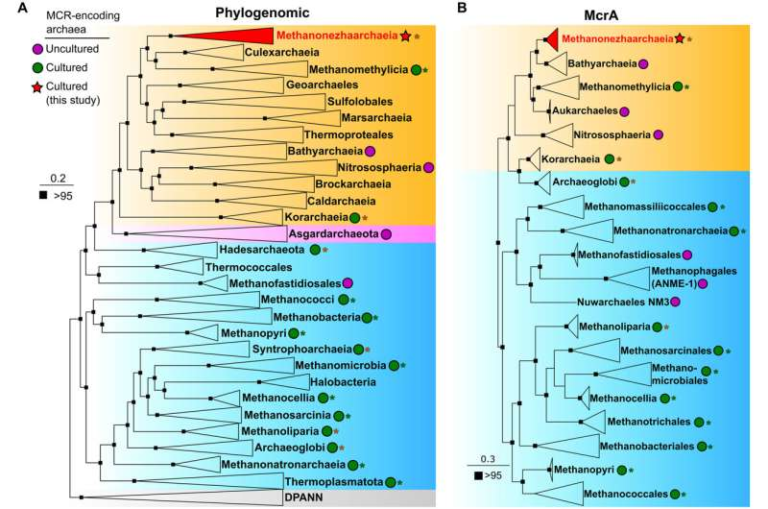
How DNA Loops Help Cells Repair Genetic Damage Faster and More Accurately
When DNA inside a cell breaks, the consequences can be serious. Broken DNA strands, especially the most dangerous kind known as double-strand breaks, can lead to mutations, cancer, or cell death if they are not repaired correctly. Scientists have long known that cells possess powerful repair systems to deal with this problem, but exactly how these systems work within the tightly packed, three-dimensional structure of DNA has remained unclear. Now, new research has uncovered an unexpected and fascinating role for DNA loops in helping cells repair genetic damage more efficiently.
DNA Damage and the Challenge of Accurate Repair
DNA carries the genetic instructions that keep cells functioning properly. However, it is constantly under attack from internal sources such as replication errors and metabolic by-products, as well as external factors like radiation and chemicals. Among the various types of damage, double-strand breaks are particularly dangerous because both strands of the DNA helix are severed at once.
To fix these breaks accurately, cells often rely on a repair pathway called homologous recombination. This process uses an intact copy of the damaged DNA sequence—usually from a sister chromatid—to guide precise repair. While homologous recombination is known to be one of the most reliable DNA repair mechanisms, scientists have struggled to understand how the cell physically finds the correct intact sequence among billions of DNA base pairs packed inside the nucleus.
The Discovery of a New Role for DNA Loops
The new study reveals that chromatin loops, which are loops formed when sections of DNA fold back on themselves, play a direct role in solving this problem. These loops are not just passive structures that help organize DNA inside the nucleus. Instead, they actively assist the cell during the homology search phase of homologous recombination.
Researchers found that when a double-strand break occurs, the cell uses these loops as guided pathways to scan along the chromosome. Rather than allowing the repair machinery to search randomly through the genome, the loops enable a more directed and efficient search for the matching DNA sequence needed for repair. In effect, the loops act like shortcuts, dramatically speeding up the repair process.
This discovery adds an entirely new function to chromatin loops, showing that they are not only important for genome organization and gene regulation, but also for maintaining genome stability.
Cohesin and Loop-Driven Chromatin Scanning
At the center of this process is a protein complex called cohesin. Cohesin is already well known for its role in holding sister chromatids together and organizing DNA into loops through a process known as loop extrusion. In this study, cohesin was shown to drive a mechanism called chromatin scanning during DNA repair.
After a double-strand break forms, cohesin helps generate loops that extend outward from the break site. These loops allow the broken DNA region to physically scan neighboring regions of the chromosome in a controlled manner. This scanning makes it much easier for the repair machinery to locate a homologous DNA sequence without wasting time exploring unrelated parts of the genome.
Importantly, the researchers observed that this loop-based scanning occurs over large genomic distances, sometimes spanning megabase-scale regions of DNA. This finding helps explain how cells can efficiently repair DNA damage even within the crowded and complex environment of the nucleus.
The Role of RAD51 in the Repair Process
Another key player in this process is RAD51, a protein that is essential for homologous recombination. RAD51 binds to single-stranded DNA at the break site and forms a filament that actively searches for a matching DNA sequence. The study shows that RAD51 works together with cohesin-generated loops to perform this search more effectively.
Instead of drifting randomly through the nucleus, RAD51-coated DNA is guided along looped chromatin regions. This coordinated action ensures that the broken DNA finds the correct repair template quickly and accurately. The combination of RAD51 activity and loop-driven scanning represents a highly efficient repair strategy that minimizes errors.
Why Cell Cycle Timing Matters
Homologous recombination mainly takes place during the S and G2 phases of the cell cycle. These are the stages when DNA has already been replicated, meaning that an identical sister chromatid is available as a repair template. The study confirms that the loop-based scanning mechanism is closely linked to these cell cycle phases.
This timing makes biological sense. By restricting homologous recombination to periods when a correct template is present, the cell reduces the risk of incorrect repairs that could introduce mutations or chromosomal rearrangements.
Why This Discovery Is Important
This research reshapes how scientists think about DNA repair in several important ways. First, it shows that three-dimensional genome architecture is not just a backdrop for cellular processes, but an active participant in them. The physical folding of DNA directly influences how efficiently repair mechanisms work.
Second, the findings have strong implications for cancer biology. Homologous recombination is often disrupted in cancer cells, either through mutations in repair genes or misregulation of chromatin structure. Understanding how DNA loops and cohesin contribute to repair could eventually help researchers identify new targets for cancer therapy or improve existing treatments that exploit DNA repair weaknesses in tumor cells.
Finally, the study opens the door to broader questions. Researchers are now interested in whether similar loop-based repair mechanisms operate in other organisms, including simpler life forms such as bacteria. If so, this could point to an evolutionarily conserved strategy for safeguarding genetic information.
A Closer Look at Chromatin Loops
Chromatin loops are formed when distant regions of DNA are brought together by protein complexes like cohesin. These loops help regulate gene expression by allowing enhancers and promoters to interact. They also help divide the genome into functional domains.
This new research adds DNA repair to the growing list of processes influenced by chromatin loops. It suggests that the same structural features that help control genes also play a vital role in protecting those genes from damage.
Homologous Recombination in Simple Terms
At its core, homologous recombination is a search-and-copy operation. The cell must find a sequence that is nearly identical to the damaged one and use it as a template for repair. The challenge lies in finding that sequence quickly and accurately within the massive genome.
By using DNA loops as scanning guides, the cell effectively narrows the search area and increases the odds of a successful repair. This elegant solution highlights just how finely tuned cellular repair systems are.
What Comes Next
The researchers behind this study plan to explore whether loop-mediated homology searching is a universal feature of DNA repair or a specialized mechanism in higher organisms. Future work may also examine how disruptions in loop formation affect genome stability and disease development.
As scientists continue to uncover the hidden roles of genome architecture, one thing is becoming increasingly clear: the shape of DNA matters just as much as its sequence.
Research paper:
https://www.science.org/doi/10.1126/science.adw1928