How RNA Storage in P-Bodies Could Help Scientists Control Cell Identity and Regeneration
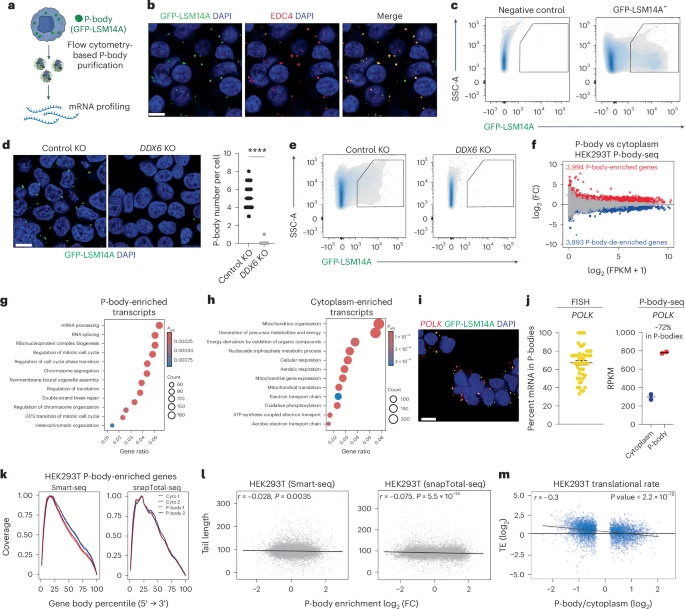
A new study published in Nature Biotechnology uncovers how tiny structures inside our cells, known as P-bodies, might hold the key to directing how cells change their identity. Researchers from Baylor College of Medicine, the University of Colorado Boulder, and several collaborating institutions have found that P-bodies don’t just store RNA — they play a decisive role in controlling what kind of cell a stem cell becomes.
In essence, P-bodies act like molecular storage lockers for RNAs — the molecules that carry genetic instructions for building proteins. What’s particularly surprising is that these P-bodies seem to hold on to RNAs from an earlier developmental stage of the cell. By keeping these RNAs tucked away, they prevent the cell from reverting to a more primitive state. But if those RNAs are released, the cell can actually revert backward, regaining earlier characteristics.
This finding changes how scientists think about cell fate, regeneration, and even infertility research. Here’s a closer look at what the study found, how it was done, and why it could be a game-changer in stem cell biology.
What the Researchers Found
The research team, led by Patrizia Pessina, Bruno Di Stefano, and Justin Brumbaugh, discovered that RNA sequestration in P-bodies plays a crucial role in determining what a cell becomes. By studying stem cells from multiple vertebrate species at various stages of development, they learned that P-bodies don’t randomly collect RNA. Instead, they selectively store RNAs that encode proteins tied to earlier cell fates.
For example, in pluripotent stem cells (which can turn into almost any cell type), the P-bodies are filled with RNAs typical of an earlier, more primitive state. When these stored RNAs are freed — either naturally or through experimental manipulation — the cell’s identity can shift backward.
This means that releasing stored RNA could effectively rewind a cell’s developmental clock. That’s a huge deal for regenerative medicine, where scientists are always looking for ways to push cells into useful states for therapy.
How They Did It
To uncover this mechanism, the team developed a new technique called P-body-seq, which allows them to isolate and analyze the RNA contents of P-bodies with high precision. Using fluorescence-activated particle sorting (FAPS), they were able to separate these microscopic structures from the rest of the cell and sequence the RNA inside.
They carried out these analyses across different species and cell types to confirm that this behavior wasn’t limited to one organism. The researchers also used genetic and biochemical experiments to disrupt P-body assembly by targeting proteins like DDX6, a key factor in maintaining P-body structure. When they did this, the cells started changing their identity — becoming totipotent-like cells or primordial germ cell-like cells (PGCLCs), which are precursors to sperm and egg cells.
In human stem cells, disabling P-body assembly led to a clear increase in markers of totipotency — genes such as DUXA, ZSCAN4, and TPRX1, which are associated with the earliest stages of embryonic development. In another experiment, disrupting P-bodies improved the efficiency of converting stem cells into PGCLCs — from less than 8% normally to more than 24% after P-body interference.
This demonstrated that manipulating these tiny RNA-holding granules can directly influence how stem cells develop, which has enormous implications for creating specific cell types in the lab.
The Role of microRNAs
Another important finding from the study involves microRNAs (miRNAs) — small, noncoding RNA molecules that regulate gene expression. The researchers discovered that certain microRNAs help drive RNA molecules into P-bodies. In other words, miRNAs act as traffic directors, guiding which RNAs get stored.
By tweaking microRNA activity, scientists might one day control exactly which RNAs are sequestered in P-bodies. That opens up the possibility of RNA-based therapies to manage cell fate. For example, manipulating specific microRNAs could push cells toward becoming nerve cells, muscle cells, or even reproductive cells, depending on the desired outcome.
Why P-Bodies Matter
P-bodies, short for processing bodies, are small compartments found in the cytoplasm of almost every eukaryotic cell. They’ve been known for years as sites for mRNA decay and storage, where untranslated RNAs are kept until the cell needs them. But this study reveals that they’re far more than passive storage sites.
Rather than just sitting around waiting to recycle old RNA, P-bodies actively participate in maintaining cell identity. They make sure certain RNAs stay silent until the right time — or possibly forever — to prevent the cell from expressing genes from an outdated stage of development.
This discovery reshapes the way scientists understand the epigenetic landscape of cells. Until recently, most attention was focused on DNA and chromatin modifications as the main regulators of cell identity. Now, RNA sequestration is emerging as a third layer of regulation, working hand in hand with transcriptional and epigenetic mechanisms to determine what a cell becomes.
Implications for Medicine and Research
The potential applications of this discovery are wide-ranging:
- Regenerative Medicine: By controlling P-body assembly, scientists could produce rare or clinically important cell types that are otherwise hard to obtain. For example, generating totipotent-like cells could help study the earliest stages of embryonic development.
- Infertility Research: Since primordial germ cells are the precursors of sperm and eggs, understanding how they form could lead to new insights into infertility and reproductive disorders.
- Cell Reprogramming: Unlocking P-body dynamics could enhance techniques for cellular reprogramming, where mature cells are converted back into stem cells or switched into different cell types entirely.
- RNA-based Therapies: Targeting the microRNAs or proteins involved in P-body formation might one day become a strategy to fine-tune gene expression in diseases linked to abnormal cell identity, such as cancer or degenerative disorders.
What Comes Next
Although these findings are groundbreaking, the researchers acknowledge that there’s still much to learn. Most of the experiments were done in cultured cells rather than whole organisms. Whether the same principles apply in complex tissues or developing embryos remains to be seen.
There’s also the question of safety and precision. P-bodies store a wide range of RNAs, and indiscriminately releasing them might have unintended effects — such as triggering the wrong developmental programs or even promoting tumor growth. Future research will focus on understanding how to target this system selectively, affecting only the RNAs relevant to a desired cell fate.
Still, this discovery provides a new and exciting angle for studying cell differentiation. It shows that RNA doesn’t just passively wait to be translated — it’s carefully managed by cellular structures that act like libraries of genetic potential, ready to be opened when the right signals come.
Understanding RNA Sequestration and Biomolecular Condensates
This study also contributes to a broader area of research involving biomolecular condensates — non-membrane compartments in cells that organize molecules through phase separation. These include P-bodies, stress granules, and nucleoli. Instead of relying on membranes, they use dynamic interactions between proteins and RNAs to form dense liquid-like droplets.
Condensate biology is an emerging field that has implications in everything from neurodegenerative diseases to viral infections. Many viruses, for example, exploit these structures to manipulate host RNA processes. The discovery that condensates directly influence cell fate transitions adds another layer of complexity — and opportunity — to how we think about cellular organization.
Limitations to Keep in Mind
Even though this work paints a compelling picture, it’s not yet clear how universal the mechanism is. Different tissues may use P-bodies differently, and there could be other condensates with overlapping or competing roles. The interplay between P-bodies, stress granules, and chromatin regulation needs deeper exploration.
Moreover, turning these findings into therapies won’t happen overnight. Manipulating P-bodies or microRNAs in living organisms requires precision tools that scientists are still developing.
Nonetheless, the concept that cell identity can be controlled through RNA storage opens a new frontier in biology — one that could redefine how we approach regenerative medicine, developmental biology, and beyond.
Research Reference:
Selective RNA sequestration in biomolecular condensates directs cell fate transitions – Nature Biotechnology (2025)