Microtubules May Be Electrical Signal Carriers Inside Neurons, According to New Physics Research
Researchers from the University of Texas at San Antonio (UTSA) have uncovered compelling new evidence that tiny structures inside nerve cells—called microtubules—may actively participate in electrical signaling. This discovery challenges long-standing assumptions in neuroscience and opens up new ways of thinking about how neurons process information, maintain health, and potentially malfunction in disease.
The findings come from a study titled Electrical Oscillations in Microtubules, published in Scientific Reports in 2025. The research was led by Marcelo Marucho, professor of physics and astronomy at UTSA and a member of its Biophysics Group, along with Md Mohsin, a doctoral researcher in physics, and several international collaborators. Their work sits at the intersection of physics, biology, and neuroscience, offering a deeper look into what happens inside neurons—not just on their outer membranes.
Shifting the Focus From Cell Membranes to the Cell Interior
For decades, neuroscience has largely focused on how neurons transmit signals across their cell membranes, using ion channels and electrical potentials to send information from one cell to another. This membrane-based signaling is undeniably crucial, but it is only part of the picture.
The UTSA team turned attention inward, to the cytoskeleton—the internal framework that gives cells their shape and structural stability. The cytoskeleton is made up primarily of actin filaments and microtubules. While these components are well known for their mechanical and transport roles, their contribution to electrical activity has often been overlooked or underestimated.
According to this new research, that oversight may be significant. The study suggests that microtubules are not electrically passive. Instead, they may behave like microscopic electrical wires, capable of generating and sustaining oscillating electrical signals over relatively long distances within the cell.
What Exactly Are Microtubules?
Microtubules are long, hollow cylindrical structures composed of repeating protein units called tubulin. They form part of the cell’s internal “skeleton,” helping maintain shape, enabling intracellular transport, and supporting cell division.
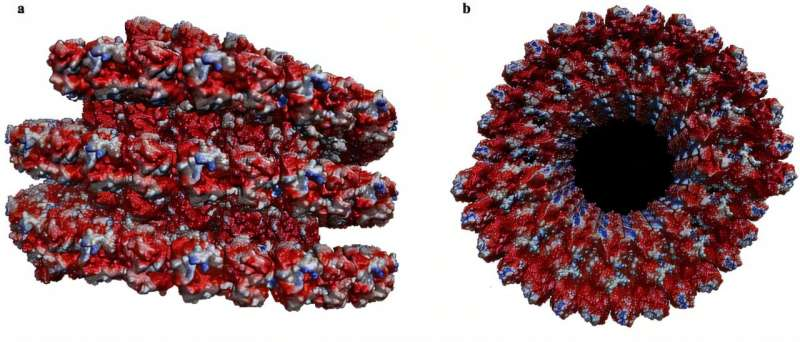
Using cryo-electron microscopy data (specifically from PDB ID: 3j2u), the researchers modeled the molecular structure and surface charge distribution of microtubules in great detail. Their models showed that microtubules have distinct regions of negative, positive, and neutral electrical charge, distributed both along their length and around their circumference. This charge pattern turns out to be critical for their electrical behavior.
Discovery of Electrical Oscillations at Brain-Relevant Frequencies
One of the most striking findings of the study is that microtubules can generate electrical oscillations at approximately 39 hertz when electrically stimulated. This frequency is especially interesting because it closely matches gamma-band brain activity, which is commonly associated with higher cognitive functions such as attention, memory, and learning.
The oscillations are not random noise. They arise from specific molecular mechanisms within the microtubule structure and appear to be self-sustaining under certain conditions. This suggests that microtubules may actively participate in intracellular electrical dynamics, rather than merely responding to signals generated elsewhere.
The Role of Nanopores and Energy Transfer
A key part of the mechanism involves tiny openings in the microtubule wall known as nanopores. These nanopores allow ions to move between the inner and outer surfaces of the microtubule.
When the microtubule is electrically stimulated, these nanopores appear to enable energy transfer across the microtubule wall, helping sustain oscillations and possibly enhancing the efficiency and duration of electrical signaling. In effect, microtubules may function as electrically active conduits, capable of coordinating biochemical reactions within different parts of the neuron.
This idea represents a major departure from the traditional view that intracellular communication relies primarily on slow diffusion of molecules. Electrical signaling along microtubules could offer a faster and more coordinated alternative.
Why This Matters for Brain Function
If microtubules do contribute to electrical signaling inside neurons, the implications are broad. Neurons are highly complex cells, and their ability to integrate signals depends on precise timing and coordination across different regions of the cell.
Electrical activity along microtubules could help regulate local biochemical reactions, synchronize internal processes, and support the overall computational capacity of neurons. This internal signaling layer may work alongside membrane-based electrical activity, adding a new dimension to how information is processed in the brain.
Potential Links to Neurodegenerative Diseases
The research also has important implications for brain health and disease. Many neurodegenerative disorders, including Alzheimer’s disease, involve disruptions to the cytoskeleton and abnormalities in microtubule stability.
If microtubules play an active role in electrical signaling, then electrical dysfunctions within these structures could contribute to neuronal failure. Understanding these processes could eventually help researchers develop new therapeutic strategies aimed at preserving or restoring proper microtubule function.
The study also hints at possible connections between cytoskeleton communication, memory formation, learning, and neuroplasticity—the brain’s ability to adapt over time. Improved understanding of these links could lead to treatments that slow memory loss or enhance cognitive resilience.
Physics Meets Biology in a Meaningful Way
One of the broader takeaways from this work is how effectively it bridges physics and biology. By applying physical models and electrical principles to biological structures, the researchers demonstrate that living systems may use unexpected mechanisms to process information.
Microtubules, long regarded as static scaffolding, are increasingly being viewed as dynamic, multifunctional components of the cell. This study strengthens that perspective by showing how their structure and charge distribution naturally lend themselves to electrical activity.
What We Already Know About Microtubules Beyond This Study
Outside of this specific research, microtubules are already known to be essential for:
- Axonal transport, moving nutrients and signaling molecules along neurons
- Cell division, where they form the mitotic spindle
- Maintaining neuronal shape, especially in long, thin axons
This new evidence of electrical behavior adds yet another layer to their importance and suggests that microtubules may be far more versatile than previously thought.
Looking Ahead
While this research does not claim that microtubules replace traditional neuronal signaling mechanisms, it strongly suggests they may complement and enhance them. Future studies will need to explore how these intracellular electrical oscillations interact with membrane-level signals and how they behave in living brain tissue.
For now, the study offers a fresh and intriguing perspective on how neurons work from the inside out—and how tiny molecular structures may have outsized influence on brain function.
Research paper: https://www.nature.com/articles/s41598-025-24920-w