MIT Researchers Develop a Gentle Enzyme-Free Method to Detach Cells From Culture Surfaces
MIT engineers have introduced a new way to detach anchorage-dependent cells from culture surfaces, and it avoids the usual enzyme-based methods that often damage cells. This fresh approach relies on alternating electrochemical redox-cycling applied to a conductive polymer nanocomposite, allowing cells to detach within minutes while keeping more than 90 percent of them alive. It’s a scientific upgrade that could reshape how labs and biomanufacturing facilities handle cell culture, especially for sensitive cells used in medicine.
What Problem This New Method Solves
Anchorage-dependent cells are cells that must attach to a surface—usually a treated dish—to survive, grow, and reproduce. They’re essential across biomedical, pharmaceutical, and cosmetic industries. However, separating these cells from the surface has long been a delicate task.
The most common technique, enzymatic detachment (often using trypsin), has some serious drawbacks. These enzymes can damage membranes and strip important surface proteins, which affects cell functionality. They also require multiple steps, consume significant amounts of reagents, and create a large amount of biological waste. Current estimates suggest that around 300 million liters of cell culture waste are produced each year from methods like this. Because many of the enzymes used are animal-derived, there are compatibility and regulatory concerns when cells are intended for therapeutic use, especially in regenerative medicine or immunotherapy.
Mechanical methods like scraping or agitation aren’t ideal either, as they can cause stress or injury to the cells. Non-enzymatic chemical methods generally don’t perform well enough for large-scale applications.
The New Enzyme-Free Electrochemical Strategy
The MIT team’s solution removes the need for enzymes entirely. Instead, the cells are grown on a biocompatible conductive polymer nanocomposite. When researchers apply low-frequency alternating voltage, the surface undergoes redox-cycling—essentially shifts in its electrochemical state. These shifts temporarily disturb cell adhesion at the interface.
The result: cells detach in a matter of minutes with minimal stress.
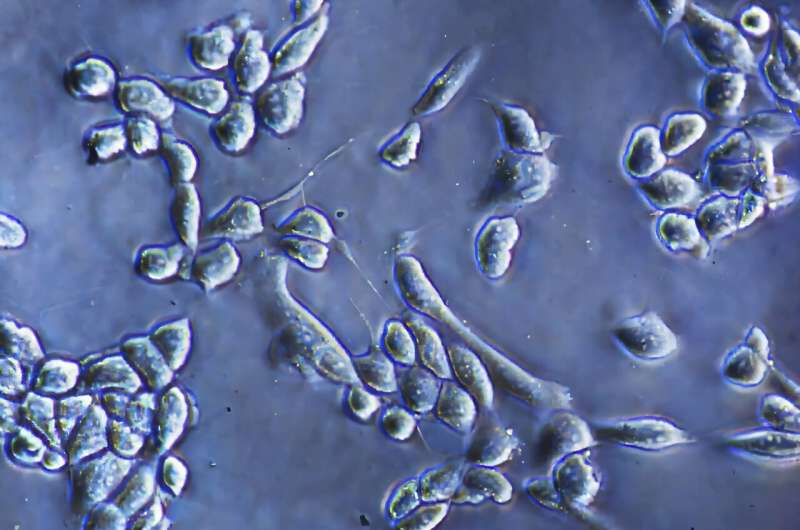
During experiments, two human cancer cell types—osteosarcoma cells and ovarian cancer cells—were tested. When the optimal frequency was applied, detachment rates jumped from 1 percent to 95 percent, all while maintaining over 90 percent viability. That makes it one of the most efficient, gentle, and rapid detachment methods tested to date.
Why This Matters for Research and Biomanufacturing
This method isn’t just a small technical improvement. It has significant implications for:
- Cell therapy manufacturing, particularly for immune cells like those used in CAR-T treatments. These cells are sensitive, and damage during detachment can make them unusable.
- Tissue engineering, where preserving cell integrity is essential for constructing functional tissues.
- Regenerative medicine, which often requires large volumes of healthy, undamaged cells.
- Drug screening and diagnostic research, where high-throughput systems depend on consistent, repeatable cell handling.
The key advantage is automation. Because the method is electrically controlled, it lends itself well to closed-loop, contamination-conscious systems. Instead of manually adding enzymes, researchers could rely on automated platforms that apply electrical signals at precise times. This greatly reduces variability and human error.
The conductive interface also allows scientists to manipulate the ionic microenvironment around cells. This opens the door to influencing ion channels, signaling pathways, and other cellular behaviors—something enzymes cannot do. Researchers see potential in linking this platform to bioelectronic systems, possibly enabling smarter drug testing or personalized therapy development.
Industrial Scaling and Practical Benefits
One of the promising aspects of the method is that it works uniformly across large surface areas. Many alternative detachment strategies fail when moving from small research plates to industrial manufacturing surfaces, but the MIT design is well-positioned for scale.
This has a few immediate benefits:
- High-throughput compatibility
- Reduced consumable waste
- Fewer animal-derived inputs
- Lower contamination risk
- More controlled and repeatable cell harvesting
Researchers involved in the project emphasize that the method is directly suitable for automated large-scale workflows, which could improve consistency in cell production for clinical and commercial applications.
Understanding Redox-Cycling in Simple Terms
Redox-cycling refers to alternating between oxidized and reduced states. When applied to the polymer nanocomposite surface, these shifts influence the ions near the cell-surface interface. Cells rely on charged interactions for adhesion, so altering the ionic environment disrupts those interactions just enough to release the cells—without harming them.
Because the voltage used is low-frequency and biocompatible, it avoids the cellular damage caused by higher-intensity electrical methods sometimes used in other biomedical tools.
Background: Anchorage-Dependent Cells and Why They’re Hard to Detach
Anchorage-dependent cells include many of the most widely used lines in research—fibroblasts, epithelial cells, stem cells, and a variety of cancer cells. Their attachment relies on integrins and extracellular matrix proteins that connect them tightly to the surface.
When removing these cells:
- Too strong a method damages them.
- Too weak a method leaves many stuck.
- Enzymes remove surface proteins, which can alter behavior in downstream experiments.
Methods like cold-shock detachment, pH-responsive polymers, and acoustic detachment have been explored over the years. While some work in small experimental setups, few offer the consistency, safety, and scalability required for clinical or industrial work.
The new electrochemical method is notable because it achieves both efficiency and gentleness, a combination that has been hard to reach with previous technologies.
Applications in Cancer Research
In the study, the researchers tested the platform using osteosarcoma (a bone cancer) and ovarian cancer cells. Both are commonly used in research for studying cancer behavior, drug responses, and metastasis. Cells detached using this method maintained their viability and functionality, which is essential for experiments that require repeated passaging or sensitive downstream analysis.
This reliability means that cancer researchers could benefit significantly from a method that preserves cell surface markers and signaling pathways—features that can be disrupted by enzymatic treatments.
Possible Future Directions
Although the platform is promising, several areas remain to be explored:
- Its behavior with primary cells, which can be more delicate than cancer cell lines.
- Long-term performance when used repeatedly in automated systems.
- Integration with multi-layered or scaffold-based culture platforms.
- Adaptation for stem cell production, where surface proteins must be preserved extremely carefully.
- Suitability for industrial-scale bioreactors that use microcarriers rather than flat surfaces.
The team believes the method can be adapted for these applications, but more studies are needed.
Research Paper Reference
Alternating Electrochemical Redox-Cycling on Nanocomposite Biointerface for High-Efficiency Enzyme-Free Cell Detachment
https://doi.org/10.1021/acsnano.5c09950