Molecular Snapshots Reveal How Our Bodies Know When It’s Too Hot
Scientists at Northwestern University have finally captured something that has puzzled researchers for years — how the body actually feels heat at the molecular level. Using some of the most advanced imaging techniques available, they’ve revealed a clear picture of one of our body’s main heat sensors, a protein called TRPM3. This discovery not only shows how our nervous system distinguishes between harmless warmth and dangerous heat, but also opens doors to developing new, non-addictive pain treatments.
The Protein That Lets Us Feel Heat
The protein at the center of this discovery, TRPM3 (Transient Receptor Potential Melastatin 3), acts like a gate sitting in the cell’s outer membrane. When it detects heat, the gate opens and allows ions—tiny charged particles—to flow into the cell. This movement of ions triggers nerve signals that the brain interprets as heat or pain.
Scientists have known for a while that TRPM3 plays a role in sensing heat, but they didn’t fully understand how it does it. Many believed that the temperature-sensing ability came from the part of the protein embedded in the cell membrane. However, this new research flips that assumption. The Northwestern team found that the heat detection actually comes from within the cell, from the part of TRPM3 that extends inside.
This means that instead of reacting only to outside temperature changes, TRPM3 also responds internally—adding an unexpected twist to how our bodies measure warmth and protect themselves from harm.
Using Cryo-Electron Microscopy to See the Unseen
Studying temperature is difficult because heat doesn’t have a shape, color, or binding site like most molecules or drugs do. To overcome this, the researchers used a high-resolution technique called cryo-electron microscopy (cryo-EM). This involves flash-freezing proteins and then taking thousands of images of them from different angles to reconstruct detailed 3D structures.
By combining cryo-EM with electrophysiology—a method that measures electrical currents passing through proteins—they could see both what TRPM3 looks like and how it behaves when activated by heat or chemicals.
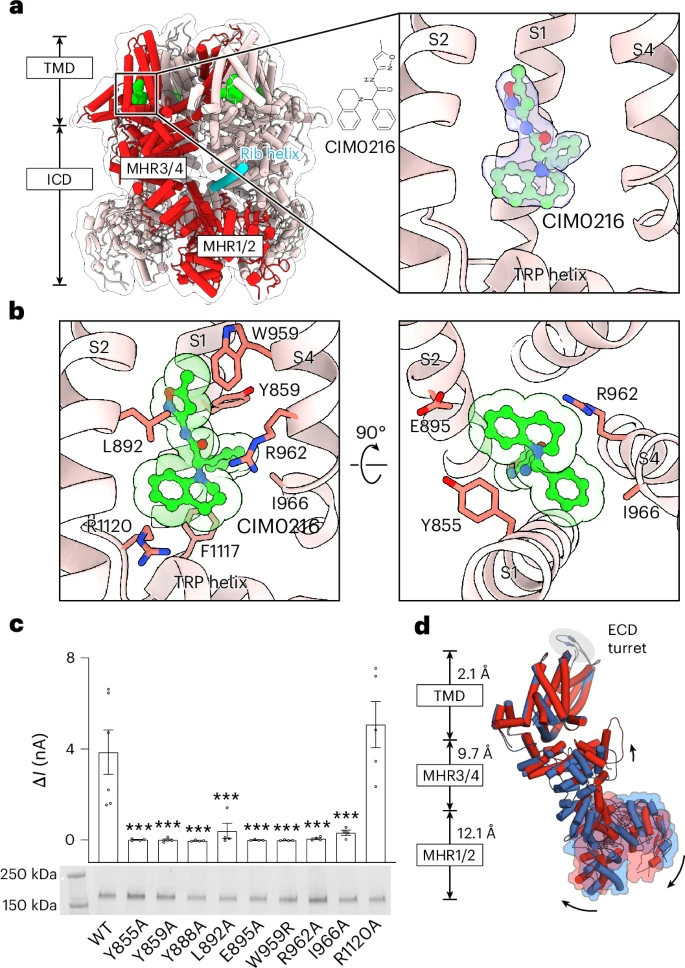
To trigger TRPM3, the team used a chemical agonist called CIM0216, which mimics the effects of heat. They also studied TRPM3’s “off” state using an epilepsy drug that binds to the protein and blocks its activation. Comparing these two states revealed which regions of the protein move or change shape when TRPM3 switches on.
They then went a step further—imaging TRPM3 at both low and high temperatures. The results showed that both heat and chemical activators caused very similar internal rearrangements, proving that they work through the same activation mechanism.
A Molecular Switch Inside the Protein
From their structural and electrical data, the researchers found that TRPM3 works like a four-part molecular switch. Each part of the protein must hold together tightly to keep the channel inactive. When exposed to heat or a chemical activator, those internal connections loosen, and the protein changes shape to let ions flow through.
In simpler terms, both heat and chemicals push the same internal button to activate TRPM3. On the other hand, certain drugs—like the epilepsy medication Primidone—can jam that button, keeping the channel locked in its inactive state.
This discovery is significant because TRPM3 exists in both sensory neurons in the skin and neurons in the brain. When it becomes overactive, it can cause pain, inflammation, or even seizures. Understanding this internal switch gives scientists a way to think about tuning TRPM3’s activity for medical purposes.
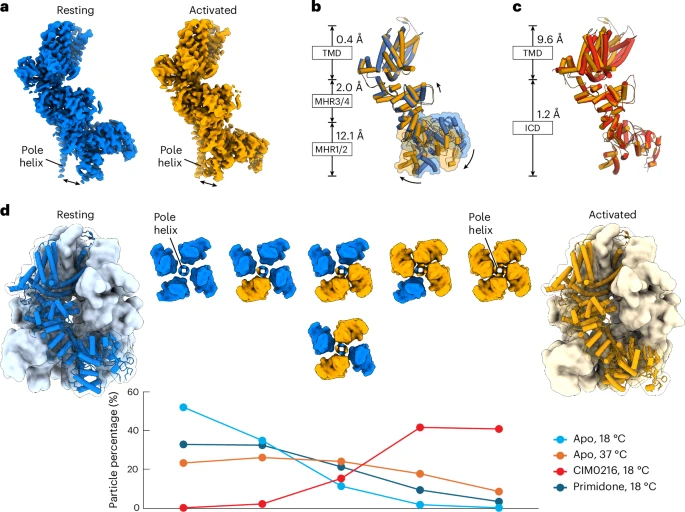
b. A structural comparison between the resting (blue) and activated (orange) apo states, aligned using the transmembrane domain (TMD), reveals clear domain shifts, with r.m.s.d. values provided for each domain and arrows indicating movement directions.
c. Comparison of the apo activated (orange) and CIM0216-bound activated (red) states, aligned via the intracellular domain (residues 1–747), highlights additional conformational changes and their r.m.s.d. values.
d. The homotetrameric TRPM3 structure is shown with four resting-state subunits (blue) and four activated-state subunits (orange). Six possible tetrameric combinations demonstrate how resting and activated subunits coexist under varying ligand or temperature conditions. The two mixed configurations (two resting and two activated subunits) are shown from the extracellular view, with resting subunits in blue and activated ones in orange.
Credit: Nature
Implications for Pain and Neurological Disorders
When TRPM3 misfires, it can lead to chronic pain or contribute to certain forms of epilepsy. Currently, many pain-relief drugs target other channels and receptors but can lead to addiction or tolerance. Because TRPM3 has such a precise activation mechanism, researchers believe that targeting this pathway could lead to non-opioid pain therapies that are safer and more effective.
This is especially relevant because TRPM3 doesn’t just respond to external burns or scalding heat—it’s also involved in body temperature regulation and inflammatory pain. If future drugs could selectively control this sensor, they might be able to reduce pain without numbing normal sensations.
Understanding the Bigger Family: TRP Channels
TRPM3 belongs to a large family of proteins known as Transient Receptor Potential (TRP) channels. These channels are scattered throughout the body and are responsible for sensing temperature, pressure, taste, and even chemicals.
Some of its more famous relatives include:
- TRPV1, the receptor that detects chili pepper’s burning sensation (capsaicin).
- TRPM8, the receptor that senses menthol and cold.
- TRPA1, which responds to irritants like wasabi or mustard oil.
Each of these channels has evolved to detect different environmental or internal stimuli, helping organisms avoid danger and maintain balance. TRPM3 is unique because it responds to heat and steroid-like compounds, and now, thanks to this research, we know exactly how that response happens.
The Tools That Made It Possible
The breakthrough came from combining two key tools:
- Cryo-electron microscopy (cryo-EM) – This method can visualize proteins at near-atomic resolution. It allowed the team to map TRPM3’s shape in both active and inactive states.
- Electrophysiology – By recording electrical currents through TRPM3, researchers could directly see how structural changes translated into function.
Using these together, the researchers created a map of TRPM3’s movement as it shifts from a closed to an open state. They even identified which amino acid regions acted as hinges or locks. This level of detail is rare in the study of temperature-sensitive channels.
What’s Still Unknown
Even with this detailed snapshot, there are still open questions. For example, researchers don’t yet know exactly how natural activators—such as pregnenolone sulfate, a steroid molecule produced in the body—bind to TRPM3. There’s also uncertainty about how TRPM3’s behavior varies across species, or how it interacts with other sensory systems in the human body.
Another mystery is whether other TRP channels use similar mechanisms for temperature sensing. Each TRP family member might have its own unique trigger and activation method, but understanding TRPM3’s structure gives scientists a valuable reference point for comparison.
Why This Discovery Matters
This research doesn’t just explain how we sense heat—it shows that the inside of a cell plays a more active role in sensory detection than previously thought. The fact that temperature sensitivity is driven from within could change how we think about pain and temperature regulation at a cellular level.
For medical science, that’s a big deal. It suggests new ways to design drugs that fine-tune internal molecular switches rather than just blocking external signals. For example, a medicine could prevent the painful overactivation of TRPM3 without shutting down normal heat sensing.
The study also demonstrates how powerful modern imaging techniques like cryo-EM have become. Decoding molecular behavior at this scale wasn’t possible just a few years ago.
The Takeaway
Our bodies rely on microscopic sensors to interpret the world around us. Among them, TRPM3 plays a key role in protecting us from dangerous heat. Thanks to this new research, we now know that this sensor doesn’t just react to surface temperature changes—it operates through an internal molecular switch deep within the cell.
By understanding exactly how that switch flips on and off, scientists can now imagine new treatments for pain and neurological disorders that work at the molecular level—precisely where the problem begins.
Research Reference:
Structural basis for agonist and heat activation of nociceptor TRPM3 – Nature Structural & Molecular Biology (2025)