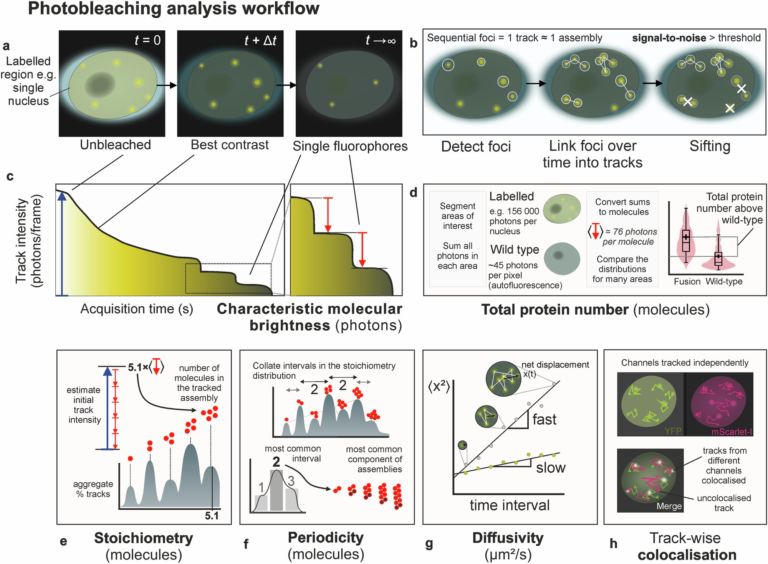
New Gene-Editing Method Uses Bacterial Retrons to Correct Large DNA Errors
A team of researchers at The University of Texas at Austin has developed a powerful new gene-editing technology that could reshape how we treat complex genetic diseases. The new approach, published in Nature Biotechnology in October 2025, retools a little-known bacterial system called retrons to fix large stretches of defective DNA with remarkable efficiency.
What Makes This Technology Different
Most current gene-editing techniques—such as CRISPR-Cas9, base editing, and prime editing—are incredibly precise but usually target single-point mutations. That’s a big limitation when it comes to diseases like cystic fibrosis, hemophilia, or Tay-Sachs disease, which can each be caused by hundreds or even thousands of different mutations across a gene.
This is where the retron-based method stands out. Instead of repairing one mutation at a time, it can replace an entire faulty DNA region with a healthy version. That means the same retron “package” can correct many variations within that region—potentially covering a whole group of patients with a single treatment.
According to the UT Austin team, this breakthrough could “democratize gene therapy” by making it financially and scientifically feasible to develop broader, more inclusive genetic treatments.
The Science Behind Retrons
Retrons were originally discovered in bacteria several decades ago. They’re genetic elements that produce small molecules of single-stranded DNA (ssDNA) from RNA using a built-in reverse transcriptase enzyme. In bacteria, retrons help defend against viral infections, but scientists have long been intrigued by their potential use in genetic engineering.
In this study, the UT team led by Jesse Buffington (graduate student) and Ilya Finkelstein (professor of molecular biosciences) found a way to engineer retrons to work efficiently in mammalian cells. By coupling retrons with the CRISPR system, they created a hybrid tool that not only cuts DNA but also provides its own repair template.
That’s a major improvement over standard CRISPR, which usually requires scientists to supply a separate donor DNA molecule to guide the repair. Retrons generate the template inside the cell, simplifying the process and boosting success rates.
Efficiency That’s 20 Times Higher
Previous attempts to use retrons for gene editing in human or animal cells were extremely inefficient—with success rates as low as 1–2%. The new method developed at UT achieves about 30% editing efficiency, a 20-fold improvement.
That’s not just a technical upgrade—it’s a potential game-changer. Higher efficiency makes it possible to think seriously about therapeutic applications, especially for diseases where only a subset of cells need to be corrected to have a major clinical impact.
The researchers also proved the system works beyond cell cultures. They successfully corrected mutations linked to scoliosis in zebrafish embryos, marking the first time retrons have been used to fix disease-causing mutations in a vertebrate.
How the Method Works
Here’s a simplified breakdown of how the process operates:
- Retrons produce ssDNA templates inside the cell using their built-in reverse transcriptase.
- CRISPR enzymes (like Cas9 or Cas12a) create a cut or nick at the target site in the genome.
- The retron-produced DNA acts as a repair blueprint, guiding the cell’s repair machinery to insert a healthy DNA sequence in place of the damaged one.
- The end result is a precise replacement of the faulty section, not just a patch.
This system allows researchers to replace long stretches of DNA—something that’s been very difficult to do efficiently until now.
Smarter and Safer Delivery
One of the persistent challenges in gene therapy is how to deliver the editing machinery safely into cells. Viral vectors can be effective but often raise immune system concerns or integration issues.
The UT team tackled this by delivering the retron system as RNA molecules encapsulated in lipid nanoparticles (LNPs). This is similar to the delivery strategy used in mRNA vaccines. LNPs help protect the RNA and ensure it gets into cells efficiently without permanent changes to the genome outside the target site.
This RNA-based delivery could be a safer, more flexible alternative to DNA-based delivery methods, reducing the risk of unwanted mutations or immune reactions.
Potential Applications
One of the first targets for this technology is cystic fibrosis (CF), a genetic disorder caused by mutations in the CFTR gene. There are over a thousand known CF-causing mutations, many of which are rare. Current treatments or gene therapies typically focus on the most common ones, leaving out patients with uncommon variants.
The retron-based approach offers a solution: it can replace a large defective portion of the CFTR gene, restoring its function regardless of which specific mutations a patient has within that segment. The researchers are already testing this on cell models that mimic CF pathology and plan to move on to airway cells derived from real CF patients.
Beyond CF, this platform could be adapted for other multi-mutation genetic disorders, including those affecting blood, muscle, or the nervous system.
Why This Matters
Gene therapy has made incredible strides, but it’s still expensive and limited in scope. Each therapy typically targets one mutation or a small group of mutations, and the development process for each is long and costly.
By creating a single retron-based template that can correct many variants, researchers hope to make gene therapy more affordable and scalable. A single FDA approval could potentially cover treatment for large groups of patients who share the same affected DNA region—even if their exact mutations differ.
This approach could bring personalized medicine closer to reality for a wider range of people, not just those with the most common genetic variants.
Challenges and Next Steps
While the results are exciting, this technology is still in its early stages. There are some challenges ahead:
- Off-target effects: Researchers need to confirm that the retron-based system doesn’t cause unwanted changes elsewhere in the genome.
- Delivery efficiency: Although LNPs are promising, reaching certain tissues (like the lungs or brain) remains difficult.
- Scalability and safety: Testing in larger animal models and eventually in humans will take time and rigorous validation.
If the method can maintain its precision and efficiency across different cell types, it could become a cornerstone of next-generation genome editing.
Broader Context: Where Retrons Fit in the Gene-Editing Landscape
Retrons add a fascinating new layer to the already powerful CRISPR toolbox. While CRISPR-Cas9 is known for its ability to cut DNA, retrons could help overcome one of CRISPR’s biggest limitations—repair.
Base editors and prime editors were developed to make small, targeted changes without cutting both DNA strands. Retrons, however, can help replace larger DNA segments, expanding the scope of what’s editable. They don’t just “fix” a letter in the DNA code; they can swap entire sentences.
Scientists also see potential for retrons in synthetic biology, functional genomics, and biotechnological manufacturing, where precise, large-scale genome edits are often required.
The Bottom Line
This research signals a major step forward in the evolution of gene editing. By harnessing a bacterial defense mechanism and turning it into a precision tool for repairing human genes, scientists have opened new doors for treating diseases once thought untreatable.
If further studies confirm its safety and versatility, retron-based gene editing could stand alongside CRISPR, base editing, and prime editing as a core technology in the future of genetic medicine.
Research Reference:
[Buffington, J.D., Finkelstein, I.J., et al. “Discovery and engineering of retrons for precise genome editing.” Nature Biotechnology (2025). DOI: 10.1038/s41587-025-02879-3**