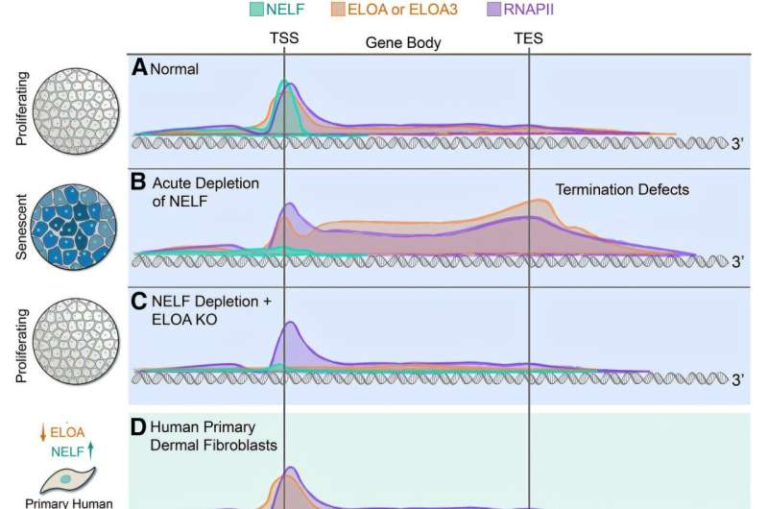
New Research Reveals How Membrane Obstacles Shape the Spread of Physical Forces in Neurons
Neurons don’t just communicate through electrical and chemical signals—they also respond to physical forces, and how those forces travel through a neuron has long puzzled scientists. A new study published in Nature Physics by researchers from ICFO – The Institute of Photonic Sciences in Spain and the University of California San Diego finally offers a detailed look at how these mechanical forces spread, and what controls their reach. The findings uncover a key role for membrane obstacles—tiny proteins and molecular structures embedded in the neuronal membrane—that either restrict or allow the propagation of tension along the cell.
This research was led by Prof. Michael Krieg at ICFO, along with Dr. Frederic Català-Castro and Dr. Neus Sanfeliu-Cerdán, in collaboration with Prof. Padmini Rangamani’s group at UC San Diego. Together, they’ve produced one of the most comprehensive models yet of how neurons transmit physical strain, combining high-precision experiments with mathematical modeling.
How Neurons Sense Physical Forces
To understand why this discovery matters, it helps to recall that neurons—and cells in general—constantly experience physical forces. From embryonic development to brain folding to the sense of touch, many biological processes depend on the ability of cells to convert mechanical stress into biochemical signals. This field of research is known as mechanobiology, and it explores how forces are felt, transmitted, and interpreted inside cells.
For decades, scientists knew that the plasma membrane (the thin, flexible layer surrounding the cell) and the cytoskeleton (the internal scaffolding) were both involved in this process. But it wasn’t clear how far mechanical tension could travel along a neuron’s membrane, or what limited its spread.
The Spark Behind the Study
The idea for this work began as a side project. The Krieg Lab had been studying how the cytoskeleton supports neuronal mechanics but started to wonder whether the membrane itself might also play an active role in carrying mechanical information. Earlier studies gave conflicting results—some suggested that tension could spread quickly across the entire membrane, while others implied it stayed localized.
To resolve this, the ICFO team turned to an incredibly precise technique known as optical tweezers, which use focused laser beams to manipulate microscopic objects and measure forces with extraordinary accuracy—down to picoNewtons and milliseconds.
The Experiment Setup
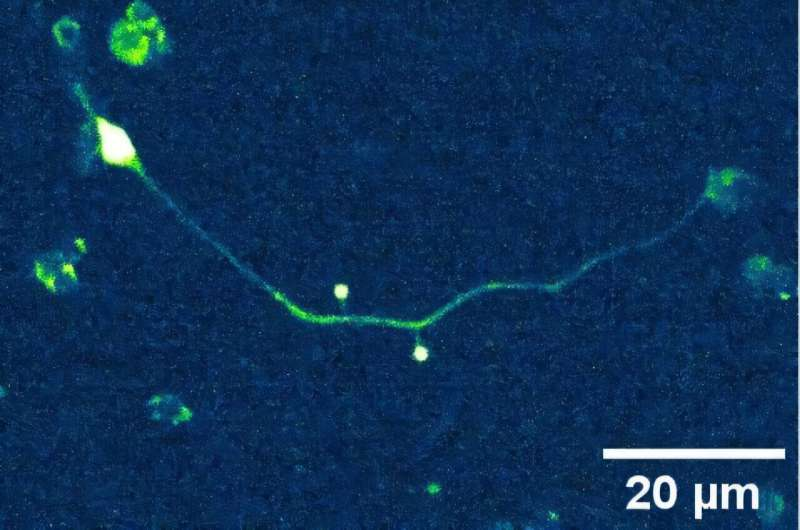
The team worked with neurons from the nematode Caenorhabditis elegans, a roundworm whose simple and well-mapped nervous system makes it an ideal model for studying basic principles of neuronal behavior.
They isolated individual neurons and attached two microspheres (tiny plastic beads) to their axons, also known as neurites. One microsphere was used to pull on the membrane using the optical tweezers, while the other measured how much of that mechanical tension traveled along the neuron’s membrane.
This setup allowed the researchers to precisely determine how fast and how far membrane tension propagates between two points on the same neuron.
What They Found
The results were striking. The speed and extent of tension propagation were not the same across all neurons. In particular, touch receptor neurons—those responsible for sensing external pressure—showed faster tension propagation than proprioceptor neurons, which detect the body’s own movements and deformations.
But what was even more interesting was that propagation depended heavily on membrane obstacles—specifically, proteins embedded in or anchored to the membrane. These obstacles don’t just slow down the movement of the membrane itself; they control how far the mechanical signal can spread.
The researchers discovered that the arrangement of these obstacles makes a big difference:
- When the obstacles are regularly spaced or arranged in an ordered pattern, they restrict the spread of tension to shorter distances.
- When the obstacles are randomly distributed, the tension can travel farther along the membrane.
This means neurons can potentially control how mechanical information spreads simply by reorganizing their membrane components.
The Role of Mathematical Modeling
To understand this in detail, the ICFO and UC San Diego teams developed 3D mathematical models that simulated how membrane tension moves in the presence of obstacles. These models took into account factors such as membrane viscosity, obstacle density, and the cytoskeletal structure underneath.
The simulations matched the experimental results closely, confirming that obstacle arrangement is a key regulator of membrane tension propagation. In some configurations, the spread of tension was limited to a few micrometers, while in others, it could extend much farther.
The models also revealed that randomness in obstacle placement helps tension bypass barriers and reach distant areas of the membrane.
Why Limiting Force Propagation Can Be Useful
At first glance, limiting how far mechanical signals travel might seem like a disadvantage—but the researchers argue the opposite. Restricting propagation can actually help neurons localize where a force is applied. If every mechanical disturbance spread across the entire cell, neurons would have trouble distinguishing one touch from another.
By keeping mechanical responses localized, neurons can more accurately pinpoint stimuli and trigger precise, area-specific reactions without activating the entire cell. In contrast, allowing tension to travel farther could be useful for neurons that need to coordinate mechanical information across longer distances—such as those involved in proprioception or whole-body movement sensing.
In other words, the membrane’s architecture allows neurons to tune their sensitivity and decide whether to act locally or globally, depending on their role.
The Importance of Obstacles
These membrane obstacles include a variety of structures—anchored proteins, ion channels, and cytoskeletal linkages—that connect the membrane to internal scaffolding like actin and spectrin networks. The study shows that these components aren’t passive—they act as mechanical regulators.
In some cases, removing or altering these structures made tension spread farther, proving that they actively shape how mechanical information is distributed. This insight also hints at possible feedback loops, where membrane tension might influence obstacle arrangement, which in turn changes how future tension spreads.
Broader Implications for Mechanobiology
This discovery adds a new dimension to the field of mechanobiology. It bridges the gap between the physical mechanics of cells and the biochemical signaling pathways they trigger. The membrane isn’t just a barrier; it’s an active, dynamic participant in how cells sense and interpret forces.
The findings could also inform studies on neuronal development, touch sensitivity, and disease conditions that alter membrane composition or cytoskeletal structure, such as neurodegenerative disorders or traumatic nerve injuries.
As external expert Dr. Eva Kreysing from the University of Cambridge pointed out, understanding how membrane tension spreads—or doesn’t—can help reveal how cells regulate critical processes such as signal transduction, growth, and morphology.
Looking Ahead
The researchers plan to dig deeper into the molecular identity of the obstacles involved and to study how they’re organized and regulated. They also aim to explore other ways cells interact with their environments, which may further influence how physical signals are transmitted.
Future work could also connect these physical findings with specific molecular mechanisms, helping scientists fully understand how forces at the cell surface eventually lead to genetic or biochemical changes inside the cell.
This study marks a major step forward in revealing the hidden mechanics of neurons, showing that even at microscopic scales, physical structure shapes how cells communicate and adapt.
Research Reference:
Català-Castro, F., Sanfeliu-Cerdán, N., Rangamani, P., & Krieg, M. (2025). Obstacles regulate membrane tension propagation to enable localized mechanotransduction. Nature Physics. https://www.nature.com/articles/s41567-025-03037-x