RNA Modifications Hold the Key to How Stem Cells Turn Into Retinal Cells

Researchers at the University of Michigan have uncovered how specific chemical changes in RNA control the way stem cells transform into retinal cells, the light-sensitive tissues responsible for vision. The findings, published in Stem Cell Reports in October 2025, open new doors for understanding retinal development, regenerative medicine, and retinal diseases such as diabetic retinopathy.
At the center of this research lies a fascinating molecule called METTL3, an enzyme that attaches small chemical tags known as methyl groups onto RNA. This modification, called m6A methylation, doesn’t change the RNA’s genetic code but influences how long the RNA lasts and how efficiently it’s turned into proteins.
What the Researchers Wanted to Know
Every cell in our body contains the same DNA, but only certain genes are active in specific cells. For a stem cell to become a retinal cell, a precise network of signals must determine which genes are turned on or off. Until now, most research has focused on DNA modifications or chromatin structure—the way DNA is packed inside the nucleus—to understand this process.
However, this study looked one layer deeper, asking how RNA modifications—chemical tags added after the RNA is copied from DNA—affect the way a cell’s identity forms. Lead researcher Rajesh C. Rao, along with first author Jing Xu and colleagues from Michigan Medicine, examined how METTL3 guides stem cells into forming retinal tissue.

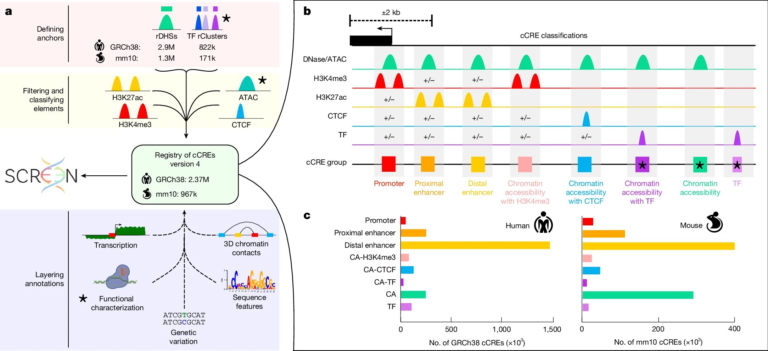
(A) Generation of rescue line: Mettl3 knockout (KO) Rx:GFP mESCs were created with Dox-inducible HA-METTL3^WT rescue. Western blot confirmed loss of endogenous Mettl3 and re-expression of METTL3^WT (HA-tag); ACTIN served as loading control (n=3).
(B) Differentiation defect: Mettl3KO mESCs failed to efficiently generate Rx:GFP^+ RPCs by day 6 (n=3).
(C) Gene expression dynamics: Heatmap analysis showed altered expression of pluripotency and eye field transcription factors in Mettl3KO cells during RPC differentiation (n=3).
(D) Catalytic requirement: Only catalytically active METTL3^WT—not mutants (APPA, W475A, N477A)—restored Rx:GFP^+ RPC differentiation in Mettl3KO mESCs, confirmed by Dox dose-dependent flow cytometry (n=3).
(E) Subcellular localization: METTL3 was detected in both cytosolic and nuclear fractions of day 6 Rx:GFP^+ RPCs, with VDAC and LSD1 as respective markers (n=3).
(F) Nuclear translocation restores function: Inducing METTL3 nuclear localization via Dex (10 μM) rescued Rx:GFP^+ expression in Mettl3KO RPCs, while cytosolic METTL3 alone did not. Dox-induced METTL3^WT also restored differentiation. Scale bar: 200 μm (n=3).
(G) Transcriptomic rescue: RNA-seq revealed that nuclear (Dex-induced) METTL3 restored downregulated gene expression in Mettl3KO organoids, whereas cytosolic METTL3 did not (p < 0.05; n=3).
Credit: University of Michigan
How They Did It
To investigate, the scientists used embryonic stem cell–derived retinal organoids. These are miniature, lab-grown versions of developing eye tissue that mimic early retinal formation. Using genetic engineering, the researchers deleted or disabled the METTL3 enzyme to observe what happens when RNA methylation is disrupted.
They discovered that without METTL3, stem cells struggled to form proper retinal structures. Specifically, METTL3 had to be present in the nucleus—the cell’s control center—to enable retinal differentiation. When METTL3 was missing or unable to add methyl groups, the cells failed to transition into Rx-positive retinal cells, a key early step in retinal development.
To map where METTL3 adds methyl groups across the entire RNA population, the team used a powerful technique called GLORI (Global RNA Interactome mapping). This allowed them to see which RNA molecules were being chemically modified during the transition from stem cell to retinal cell.
They then integrated this data with RNA sequencing, ATAC-seq (which measures chromatin accessibility), and ChIP-seq (which tracks protein-DNA interactions). These combined approaches provided a comprehensive view of how RNA modifications intersect with gene expression and chromatin structure.
A Surprising Discovery
The researchers uncovered something unexpected: chromatin accessibility and transcription became uncoupled when METTL3 was removed. Normally, when DNA becomes more accessible, the corresponding genes are turned on. But in this case, even though chromatin opened up, the genes weren’t necessarily active.
This means that RNA methylation can act as a separate layer of control, influencing gene expression independently of chromatin state. It’s a major insight because it challenges the long-held assumption that chromatin accessibility alone dictates gene activity.
The Role of Six3 and RNA Stability
One of the most critical genes affected by RNA methylation was Six3, which plays a crucial role in transforming stem cells into retinal cells. The team found that METTL3 added methyl groups to the 3′ end of the Six3 RNA molecule. This modification helped stabilize the RNA, allowing the cell to produce the necessary proteins for retinal development.
When they used CRISPR-based RNA editing tools to remove the methylation marks from Six3 RNA, the molecule became unstable, leading to a failure in retinal differentiation. This showed that RNA stability—controlled by these chemical tags—is vital for proper gene regulation.
Further experiments revealed that inhibiting the Ythdf family of genes, which read and interpret m6A tags on RNA, had the same blocking effect as deleting METTL3. This established a METTL3–Ythdf regulatory pathway that ensures stem cells can mature into retinal tissue.
Beyond DNA: Understanding the RNA Epigenetic Layer
This study is the first to describe the mechanisms of RNA epigenetics in retinal cell development. While traditional epigenetics focuses on DNA and histones, epitranscriptomics—the study of RNA modifications—is emerging as a crucial control system in how cells make identity decisions.
In simple terms, DNA tells the cell what’s possible, but RNA modifications help decide what actually happens. By controlling the lifetime and efficiency of RNA messages, cells can fine-tune which proteins are produced and in what amounts.
Implications for Retinal Diseases
The research team also pointed out an intriguing link between RNA modifications and metabolic stress. They noted that high sugar levels can alter RNA methylation patterns. Since the retina is extremely sensitive to metabolic imbalances, this raises questions about how RNA methylation might contribute to diabetic eye diseases.
If RNA modifications are disrupted under high-glucose conditions, it could explain some of the molecular damage seen in diabetic retinopathy—a leading cause of blindness worldwide. Understanding these mechanisms could lead to new therapeutic targets for preventing or repairing retinal damage.
Potential for Regenerative Medicine
These findings also have exciting implications for regenerative medicine. If scientists can control RNA modifications precisely, they might improve the efficiency of turning stem cells into retinal cells in the lab. That would help produce better-quality cells for transplantation therapies in patients with retinal degeneration.
In addition, manipulating the METTL3–Ythdf pathway could allow researchers to regulate how fast or how completely stem cells mature into specific retinal types, such as photoreceptors or retinal ganglion cells. This could make a big difference in developing cell-based treatments for blindness.
What We Still Don’t Know
Although the study provides a detailed look at RNA methylation’s role in retinal development, some questions remain open. For instance, it’s still unclear how METTL3 influences chromatin accessibility without directly changing gene transcription. The connection between RNA modifications and chromatin organization may involve additional proteins or signaling pathways that haven’t been discovered yet.
Also, the experiments were primarily conducted in lab-grown retinal organoids. While these are reliable models, verifying the same results in living animals or human tissues will be important. Future studies could use conditional METTL3 knockouts in mice to see whether the same RNA modification patterns appear in natural retinal development.
Finally, researchers want to explore whether other RNA modification enzymes besides METTL3 play roles in different stages of eye formation. METTL3 may be part of a larger network of RNA modifiers that collectively shape cell identity.
Why This Research Matters
The discovery that RNA modifications can decouple chromatin changes from transcription marks a turning point in developmental biology. It expands the definition of gene regulation beyond DNA and histones, introducing a flexible new layer of control that can respond to cellular conditions and environmental cues.
In the long run, understanding this mechanism could impact not only vision science but also other areas of medicine. Similar RNA methylation processes operate in the brain, heart, and pancreas, influencing cell fate decisions and disease outcomes.
By mapping how RNA modifications guide development, scientists are getting closer to understanding the blueprint of life in motion—how the same DNA can create such diverse cell types, and how small chemical tags can tip the balance between health and disease.