Scientists Create a Safe and Reversible Gene Switch That Uses a Common Antiviral Drug

Researchers at Weill Cornell Medicine have unveiled a groundbreaking technology that allows scientists to control gene activity with precision — and without harming cells. The new system, called Cyclone, introduces a way to turn genes on or off inside living cells using a non-toxic small molecule, the antiviral drug acyclovir. This invention could reshape how scientists study genes, model diseases, and even develop safer gene therapies.
The details of this work appear in the journal Nature Methods, under the title “A portable poison exon for small-molecule control of mammalian gene expression”. The research was led by Dr. Samie Jaffrey, the Greenberg-Starr Professor of Pharmacology at Weill Cornell Medicine, with Qian Hou, a Ph.D. candidate in Jaffrey’s lab, as the first author.
What Exactly Is a Gene Switch?
A gene switch is a tool that lets researchers control when and how much a gene is active. In biology, genes are like instructions for making proteins, and by controlling these instructions, scientists can observe what happens when a gene is silenced or reactivated. It’s a bit like having a dimmer switch for your genes — turn it up to study what a protein does when abundant, or turn it down to see what happens when it’s missing.
Traditional systems, such as the tetracycline-controlled transcriptional activation (Tet-On/Tet-Off) systems, rely on antibiotics like tetracycline or doxycycline to toggle gene activity. These have been useful but come with serious drawbacks: they can be toxic to cells, cause unwanted side effects, and often disturb RNA transcripts, meaning the final protein product doesn’t always match what nature intended.
Cyclone changes this game. Instead of using an antibiotic, it relies on acyclovir, a drug commonly prescribed to treat herpes infections, which is known for being safe, well-tolerated, and non-toxic, even at high doses.
How the Cyclone System Works
Cyclone is based on a clever natural concept called a poison exon. In simple terms, a poison exon is a piece of DNA within a gene that, when included during the process of making RNA, disrupts the production of the final protein. The presence of this exon effectively shuts off the gene.
Here’s where the innovation comes in: the Weill Cornell team engineered a synthetic poison exon cassette — an “intron–poison exon–intron” module — that can be inserted into any target gene. This engineered sequence acts as a built-in off switch. When no drug is present, the gene remains silenced because the poison exon interrupts its message.
When acyclovir is added, it binds to a specially designed RNA structure in the cassette. This interaction causes the cell’s machinery to skip the poison exon, allowing the gene to produce its normal protein again. Once the drug is removed, the gene turns off again.
This process is reversible, tunable, and safe, giving researchers full control over gene expression without altering the gene’s final protein product.
The Science Behind the Innovation
Cyclone’s design revolves around controlling RNA splicing, the process by which cells cut and paste RNA segments before they are translated into proteins. The system integrates a small RNA aptamer — a structure that binds to acyclovir with a high affinity (about 1.3 micromolar dissociation constant) — allowing precise responsiveness to the drug.
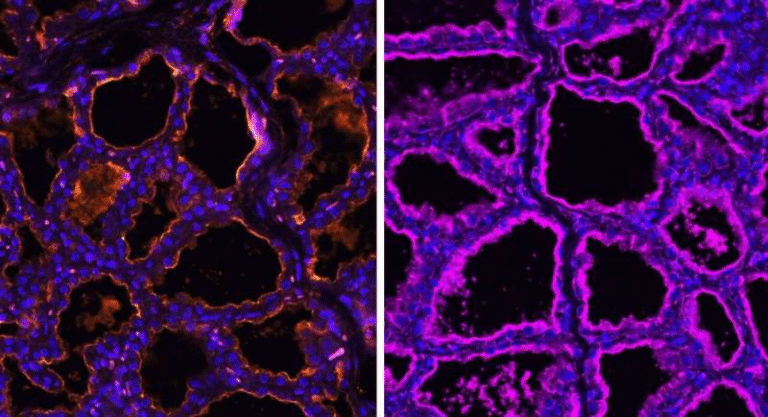
In experiments, inserting the Cyclone cassette into a luciferase reporter gene (a standard test gene) reduced its expression by about 92% in the “off” state. When acyclovir was added, expression was restored — sometimes boosted to 300% of normal levels, depending on the dose.
Researchers demonstrated that Cyclone works not only with artificially introduced genes but also with natural genes already present in cells, which is a major step forward. They also created a variant called Pac-Cyclone, which makes it easier to introduce this control mechanism into native genes using gene-editing tools like CRISPR.
Because the final RNA transcript is indistinguishable from the natural version after splicing, Cyclone is described as “traceless” — meaning it leaves no unwanted genetic scars behind.
Why Acyclovir Is a Smart Choice
Acyclovir’s selection as the control molecule was intentional. This antiviral drug is one of the most widely used and safest small molecules known in medicine. It’s been prescribed for decades to treat viral infections such as herpes simplex and varicella-zoster, and its pharmacological properties are well understood.
By using acyclovir, researchers avoid the cytotoxicity and side effects associated with many chemical inducers. Since it’s already approved for human use, this also opens up possibilities for clinical applications — especially for gene therapies, where safety is critical.
Moreover, the system could, in theory, be adapted to use other small molecules by swapping the acyclovir-binding element for another aptamer. This modularity means future versions of Cyclone could respond to different drugs, enabling scientists to control multiple genes at once with different compounds.
What Makes Cyclone Different
The Cyclone system improves on previous gene control tools in several key ways:
- Safety: Uses a well-tolerated antiviral drug instead of antibiotics or hormones.
- Reversibility: Gene expression can be turned on or off repeatedly, with no lasting effects.
- Precision: The resulting RNA and protein are identical to their natural versions.
- Tunability: Expression levels can range from 0% to 300% depending on drug concentration.
- Versatility: Works with both engineered and natural genes.
These features make Cyclone a major leap forward in gene regulation technology.
Real-World Applications and Future Potential
Cyclone’s impact could be felt across many areas of biological and medical research. In the lab, scientists could use it to study how genes function in diseases like cancer, neurodegeneration, or developmental disorders. By selectively activating or silencing a gene, researchers can observe its exact role in a living system.
In the clinic, Cyclone’s potential is even more exciting. Gene therapies often face the challenge of safety — once a therapeutic gene is turned on, there’s little control over its activity. With Cyclone, doctors could potentially use a reversible switch to regulate therapeutic genes in patients, turning them off if side effects appear or fine-tuning their activity levels.
This could lead to smarter and safer gene therapies, where treatments are not just inserted and forgotten but actively managed like any other medication.
Broader Context: The Future of Controllable Gene Expression
Gene regulation tools are at the heart of modern synthetic biology — a field that combines genetics and engineering to design biological systems with new functions. The ability to control gene activity underpins everything from cell-based therapies to biosensors and programmable organisms.
Systems like Cyclone could also merge with CRISPR-based technologies, allowing not just editing of genes but also dynamic control of how and when those edited genes are expressed.
Imagine a world where engineered immune cells could be temporarily deactivated during side effects, or where therapeutic proteins could be produced only when needed — this is the kind of precise control Cyclone points toward.
Challenges and Next Steps
Despite its promise, Cyclone still faces some hurdles. The technology relies on inserting the cassette into a gene, which means gene-editing methods like CRISPR or viral vectors are required — not yet routine in all cell types. Also, while acyclovir is safe in humans, its effects in this new genetic context need long-term testing.
Future work will explore whether similar poison exon systems can be tuned for different ligands, allowing a set of independent switches within the same cell. Such innovations could bring synthetic biology closer to achieving complex, multi-gene control networks — a key milestone in advanced genetic engineering.
Final Thoughts
Cyclone represents one of the most promising breakthroughs in gene regulation in recent years. Its safety, precision, and flexibility make it a valuable tool for researchers and a potential cornerstone for future gene therapies. By taking inspiration from the cell’s natural mechanisms and combining it with a common antiviral drug, the Weill Cornell team has created a system that could redefine how we interact with our genes — safely and reversibly.