UCLA Scientists Discover How Pseudomonas Bacteria Follow Sugar Trails to Form Infections

A team of researchers from the California NanoSystems Institute (CNSI) at UCLA has uncovered how one of the most stubborn and dangerous bacteria, Pseudomonas aeruginosa, figures out where to cluster and form infections inside the human body. This new finding, published in Nature Microbiology (2025), sheds light on how these bacteria use mechanical sensing to detect and follow sugar trails, eventually building tough biofilm communities that resist antibiotics.
Understanding Pseudomonas aeruginosa and Why It Matters
Pseudomonas aeruginosa is an opportunistic pathogen — a microbe that takes advantage of weakened immune systems or damaged tissues. It’s a major threat to patients with cystic fibrosis, those on ventilators, and anyone recovering from severe COVID-19 infections. The World Health Organization lists Pseudomonas among the most antibiotic-resistant bacteria in the world.
When Pseudomonas cells attach to a surface, they begin to produce a sticky material made up of exopolysaccharides (EPS) — sugar-based polymers that hold them together. This allows the bacteria to form biofilms, slimy communities that coat surfaces. Inside a biofilm, the bacteria are up to 1,000 times more resistant to antibiotics than free-floating cells. This makes infections extremely difficult to treat, especially in hospital settings.
But until now, scientists didn’t fully understand how these bacteria decide where to attach and start forming these persistent colonies.
How the Study Was Conducted
The UCLA-led team, working with bioengineering and microbiology experts, designed an experiment that mimicked the bacteria’s natural environment. They created artificial sugar trails on a surface using a synthetic version of the same sugars produced by Pseudomonas itself.
This setup allowed the researchers to observe how individual bacteria behaved as they moved across the patterned surface. They used genetic engineering to modify certain bacterial strains so some could not produce or sense sugars. They also used advanced imaging and cell-tracking techniques to follow how the bacteria responded to different sugar signals.
To measure how the bacteria made decisions, the team monitored two important chemical signals inside the cells — molecules called cyclic di-GMP and cyclic AMP. These molecules control whether a bacterium continues to swim freely or starts to settle down and form a biofilm.
What the Researchers Found
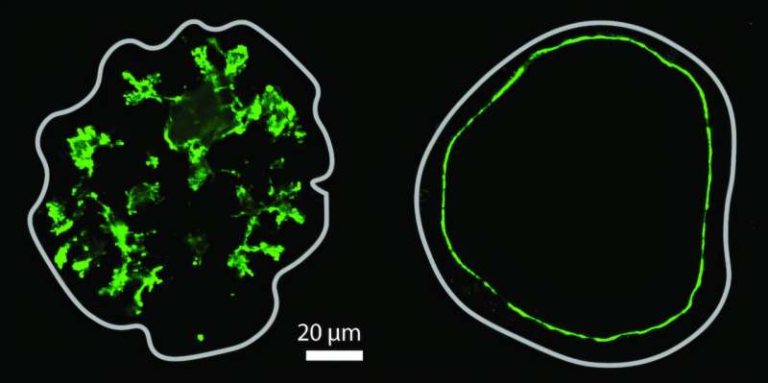
The scientists discovered that Pseudomonas can detect and follow sugar trails left behind by its own kind. When one bacterial cell moves across a surface, it secretes polysaccharides that form a trail. Other cells that arrive later can sense these trails and follow them, effectively “walking in the footsteps” of earlier bacteria.
The key to this sensing process lies in the bacteria’s type IV pili — tiny, hair-like appendages on their surface. Normally, pili are used for twitching motility, a kind of crawling movement across surfaces. But this study found that the pili also act as mechanical sensors.
Here’s how it works:
- The pili attach to the sugar molecules on the surface.
- As the bacterium pulls on these molecules, it feels mechanical resistance — a tug that signals it has found something solid and worth holding onto.
- This physical force triggers chemical changes inside the cell.
- The internal signals tell the bacterium to start making more sticky sugars and slow down its movement.
In other words, the pili don’t just help the bacteria move — they also help them decide when to stop moving and start building.
The process combines both chemical sensing (detecting specific sugar molecules) and mechanical sensing (feeling the physical strength of the bond). This dual mechanism explains how Pseudomonas switches from being an exploring, free-swimming cell to becoming part of a stable, antibiotic-resistant biofilm.
Why This Discovery Is Important
Understanding how Pseudomonas detects sugar trails could open up new ways to combat bacterial infections. Right now, antibiotics have limited effect on bacteria embedded in biofilms. But if scientists can disrupt the sensing process, they might prevent biofilms from forming in the first place.
Researchers suggest that targeting the pili, adhesins (proteins that help bacteria stick to surfaces), or the mechanical signaling pathway could make bacteria more vulnerable. By tricking the bacteria into thinking they have no surface to attach to, doctors could keep them in their more antibiotic-susceptible swimming form.
The findings could also help address industrial and environmental problems caused by bacterial biofilms. These slimy layers often clog water pipes, filters, and even ship hulls, costing industries billions each year in cleaning and maintenance. If scientists can create surfaces that mimic “empty space” or that bacteria simply can’t sense properly, it might stop biofilms from forming altogether.
The Science Behind Biofilm Formation
Biofilm formation happens in several stages:
- Initial attachment – individual cells attach loosely to a surface using pili or flagella.
- Irreversible attachment – the bacteria start producing sticky exopolysaccharides that cement them in place.
- Microcolony formation – the bacteria multiply and form small clusters.
- Biofilm maturation – the clusters grow into complex 3D structures surrounded by EPS.
- Dispersal – some cells break free and move elsewhere to start new biofilms.
Pseudomonas aeruginosa uses several polysaccharides in this process, but one of the most important is Psl, which creates the sugar trails that the new study investigated. Another key component is CdrA, a protein that binds to Psl and helps cells stick together more tightly.
The interaction between Psl, CdrA, and the type IV pili gives Pseudomonas a powerful toolkit for detecting surfaces, sensing signals, and building resilient communities.
Broader Impact and Future Research
The study opens the door for multiple lines of research. Scientists now plan to investigate how different surface shapes or materials influence the bacteria’s sensing abilities. They also want to test whether other species use similar mechanisms — since many harmful bacteria, such as Staphylococcus aureus or E. coli, also form biofilms.
In medicine, researchers hope this discovery could help treat chronic infections in lungs, wounds, and medical devices like catheters and ventilators. For instance, developing drugs that interfere with mechanical sensing might make existing antibiotics much more effective.
The UCLA team is also exploring how long these sugar-sensing signals last and whether they can be passed down through generations of bacteria. This could explain how biofilms maintain their structure and behavior even as individual cells die and new ones replace them.
The Bigger Picture
This research doesn’t just solve a microbiological puzzle — it also changes how scientists think about bacterial intelligence. Even though bacteria are single-celled organisms, they can collectively sense, communicate, and coordinate their actions through physical and chemical cues.
It’s a reminder that bacteria are not passive. They don’t just drift around waiting to infect something — they actively explore, detect, and adapt to their environments in surprisingly complex ways.
Understanding that bacteria can “feel” their surroundings adds a new dimension to microbiology. It also highlights why developing new anti-biofilm strategies is crucial as antibiotic resistance continues to rise.
As one of the researchers put it, this is the first time scientists have seen how bacteria translate mechanical forces into internal chemical decisions — a process that could reshape how we approach everything from infection control to materials science.