Under Pressure: How Scientists Used a Synchrotron to Reveal Hidden Differences in DNA Packaging
When we think about DNA, most of us imagine that familiar twisted ladder shape — the double helix. But inside our cells, DNA isn’t floating freely. It’s packed, folded, and coiled in astonishingly complex ways. About two meters of DNA fit inside a nucleus that’s just a few micrometers wide. How does that happen? The secret lies in structures called nucleosomes, which act like spools around which DNA winds.
A new study from the University of Pennsylvania, in collaboration with scientists at the Cornell High Energy Synchrotron Source (CHESS), has taken an unusual approach to studying these nucleosomes: they literally squeezed DNA under extreme pressure. The research, published in the journal Chromosome Research (2025), reveals how different types of nucleosomes behave when subjected to conditions thousands of times greater than normal atmospheric pressure — and what that means for the way life organizes its genetic material.
The Science Behind the Squeeze
At the core of this work is Dr. Kushol Gupta, a structural biologist at the University of Pennsylvania. He wanted to understand how DNA packaging structures respond when stressed — not chemically, but physically. To do this, his team turned to high-pressure small-angle X-ray scattering (HP-SAXS), a method available at CHESS’s bioSAXS beamline (ID7A1).
This beamline is one of the few in the United States capable of performing high-pressure scattering experiments on biological samples, making it perfect for testing how DNA behaves under extreme conditions.
The setup involved a custom-built hydrostatic pressure cell, designed by Durgesh Rai and Richard Gillilan, that can reach pressures up to 400 megapascals (MPa). To put that in perspective, that’s roughly 4,000 times the pressure we experience at sea level, or about four times the pressure at the bottom of the Mariana Trench, the deepest point in Earth’s oceans.
What Are Nucleosomes and Why They Matter
Each nucleosome is made up of DNA wrapped around a group of proteins known as histones. Think of them as tiny reels that keep DNA organized and protected. These histones aren’t all the same — and that’s crucial.
The canonical nucleosome contains a standard version of the protein histone H3, but in a special part of the chromosome called the centromere, this protein is replaced with a variant known as CENP-A. The centromere is where chromosomes attach to the spindle fibers during cell division, so it’s a mechanically demanding region.
Researchers have long suspected that nucleosomes containing CENP-A are physically different — perhaps stronger or more stable — but observing these differences in realistic, solution-based conditions has been challenging. That’s where Gupta’s high-pressure experiment came in.
How the Experiment Worked
The team prepared two sets of nucleosome samples:
- Conventional nucleosomes containing histone H3.
- Centromeric nucleosomes containing CENP-A.
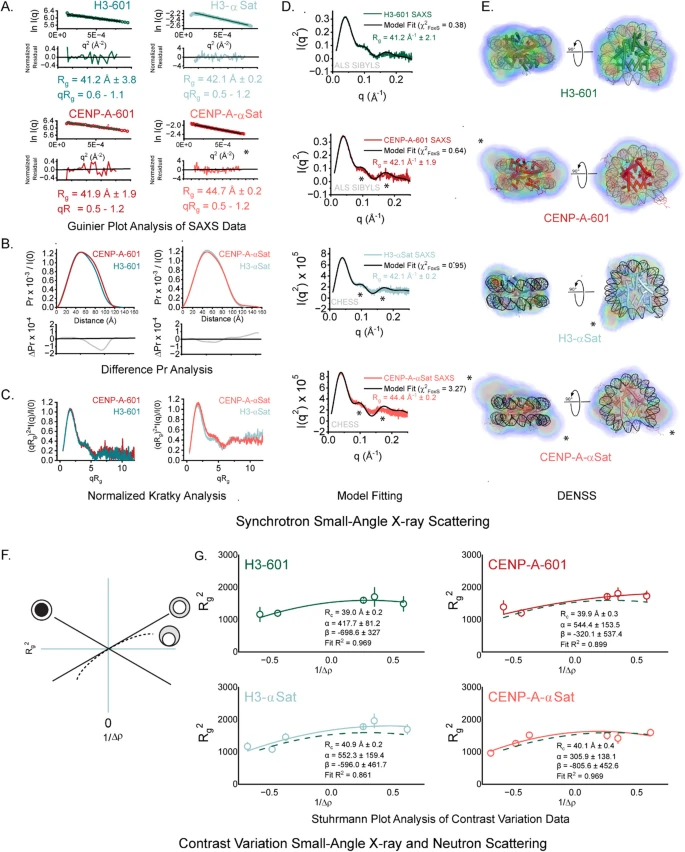
They then gradually increased the pressure on these samples — up to 300 MPa — while recording how their structure changed using X-ray scattering. This technique provides information about the shape and size of molecules in solution by measuring how X-rays scatter off them.
Under pressure, biological molecules can compress, twist, or partially unfold, and by monitoring how the scattering pattern changed, the team could see how each type of nucleosome responded.
What They Found
The results were striking.
As pressure increased, conventional nucleosomes began to partially unravel — the DNA started loosening from the histone core. In contrast, centromeric nucleosomes were far more resilient, holding their structure together even at high pressures.
When the pressure was released, something surprising happened: both types of nucleosomes returned almost perfectly to their original shapes. This meant the deformation was reversible — a clear sign that the structure of chromatin is elastic, not brittle.
Gupta described this as a kind of “molecular stress test”, showing that chromatin can handle extreme conditions and bounce back afterward. It also demonstrated that CENP-A nucleosomes are built to endure more stress, fitting their crucial role at the heart of chromosomes during cell division.
Why Pressure Is a Powerful Tool
Most biological experiments involve altering molecules through mutation, chemicals, or heat. Pressure, on the other hand, doesn’t permanently change the sample — it just perturbs it.
This makes pressure-based experiments particularly valuable for studying fragile structures like chromatin, because they let scientists observe natural responses without damaging the molecules.
Gupta’s work shows that applying pressure can reveal hidden features of biomolecular organization — how stable certain bonds are, how flexible DNA wrapping can be, and what happens to proteins when the forces that hold them together are strained.
The Bigger Picture: Chromatin Mechanics
Chromatin — the mix of DNA and proteins that fills our cell nuclei — is not just a passive storage system for genetic information. It’s an active, dynamic material that constantly adjusts to allow gene expression, replication, and repair.
By understanding how chromatin components behave under stress, researchers can learn how gene regulation might be influenced by mechanical forces — both natural and environmental.
This has implications beyond the lab:
- Cell division: How chromosomes maintain their shape during the physical strain of mitosis.
- Extreme environments: How life can persist under enormous pressure, such as in deep-sea organisms.
- Genome integrity: How cells protect their DNA when the nucleus is compressed, as happens during cell migration or cancer metastasis.
Expanding the Technique
The same HP-SAXS technique used here isn’t limited to DNA. Gupta’s lab is already applying it to other biological systems — including vaccine-related particles, protein-protein interactions, and enzyme-ligand binding.
Each of these applications benefits from being able to see how molecules adapt and recover when physically stressed. As more labs adopt this approach, high-pressure scattering may become a standard method for exploring the hidden dynamics of life’s molecular machinery.
Why Centromeric Nucleosomes Are So Special
The study reinforces the idea that CENP-A nucleosomes have distinct mechanical properties. Their ability to resist deformation could be key to centromere stability — ensuring chromosomes don’t break apart or mis-segregate during cell division.
CENP-A nucleosomes also create a foundation for building the kinetochore, a protein complex that physically links chromosomes to the mitotic spindle. If these nucleosomes were less stable, the entire process of cell division could fail, leading to chromosomal abnormalities.
In short, the mechanical toughness of CENP-A nucleosomes might be one of the unsung heroes of cellular life — a small but crucial adaptation that keeps our genetic material safe every time a cell divides.
A New Way to Look at DNA Packaging
This research doesn’t just add to what we know — it changes how we can study DNA packaging. By using pressure as a clean, controllable variable, scientists can explore new frontiers in molecular biology without relying on destructive methods.
It also hints at a broader idea: our DNA is not only a chemical code but also a physical structure, one that must endure, flex, and recover in a constantly changing cellular world. Understanding that mechanical resilience might help unlock new insights into how life maintains order under stress.
Research Reference:
Kushol Gupta et al., Solution conformational differences between conventional and CENP-A nucleosomes are accentuated by reversible deformation under high pressure, Chromosome Research (2025). DOI: 10.1007/s10577-025-09769-z