Understanding the Link Between Nucleotide Metabolism and Chromatin Assembly
Scientists have long known that for a cell to successfully copy and protect its DNA, two things must happen in parallel. First, the cell needs a steady supply of nucleotides, the basic building blocks of DNA. Second, it must produce enough histones, the specialized proteins that DNA wraps around to form chromatin. What has remained unclear for decades is how these two essential processes stay coordinated. A new study from Northwestern Medicine, published in Molecular Cell, now reveals that they are far more tightly connected than previously believed.
This research uncovers a previously unknown molecular link between nucleotide metabolism and chromatin assembly, showing that enzymes responsible for starting nucleotide synthesis also play a direct role in preparing histones for incorporation into chromatin. The findings add an important new layer to our understanding of how cells maintain genome stability during DNA replication and cell division.
Why Chromatin Assembly Matters
Inside the nucleus, DNA is not left as a loose, fragile thread. Instead, it is wrapped around histone proteins to form chromatin, a compact structure that protects genetic material and regulates gene activity. Proper chromatin assembly is critical during DNA replication, when the genome must be duplicated quickly and accurately.
If DNA is copied without enough histones available, large stretches of naked DNA are exposed. This dramatically increases the risk of DNA damage, replication stress, and genomic instability. These problems are closely linked to cancer, aging, and various genetic disorders. For this reason, cells must carefully balance DNA synthesis with histone production and deposition.
The Longstanding Puzzle of Coordination
For many years, researchers assumed that nucleotide production and histone supply were regulated independently. Nucleotides are generated through metabolic pathways, while histones are produced by ribosomes and then guided to DNA by histone chaperone proteins. Although both processes are essential for replication, there was no clear mechanism explaining how the cell ensures they proceed at matching speeds.
The Northwestern Medicine study directly addressed this question by focusing on phosphoribosyl pyrophosphate synthetases, commonly known as PRPS enzymes.
PRPS Enzymes and Their Unexpected Role
PRPS enzymes are best known for catalyzing the first and rate-limiting step in nucleotide biosynthesis. Without them, cells cannot efficiently produce the nucleotides needed to build DNA and RNA. In humans, these enzymes function as part of a larger complex that includes PRPS-associated proteins, such as PRPSAP1.
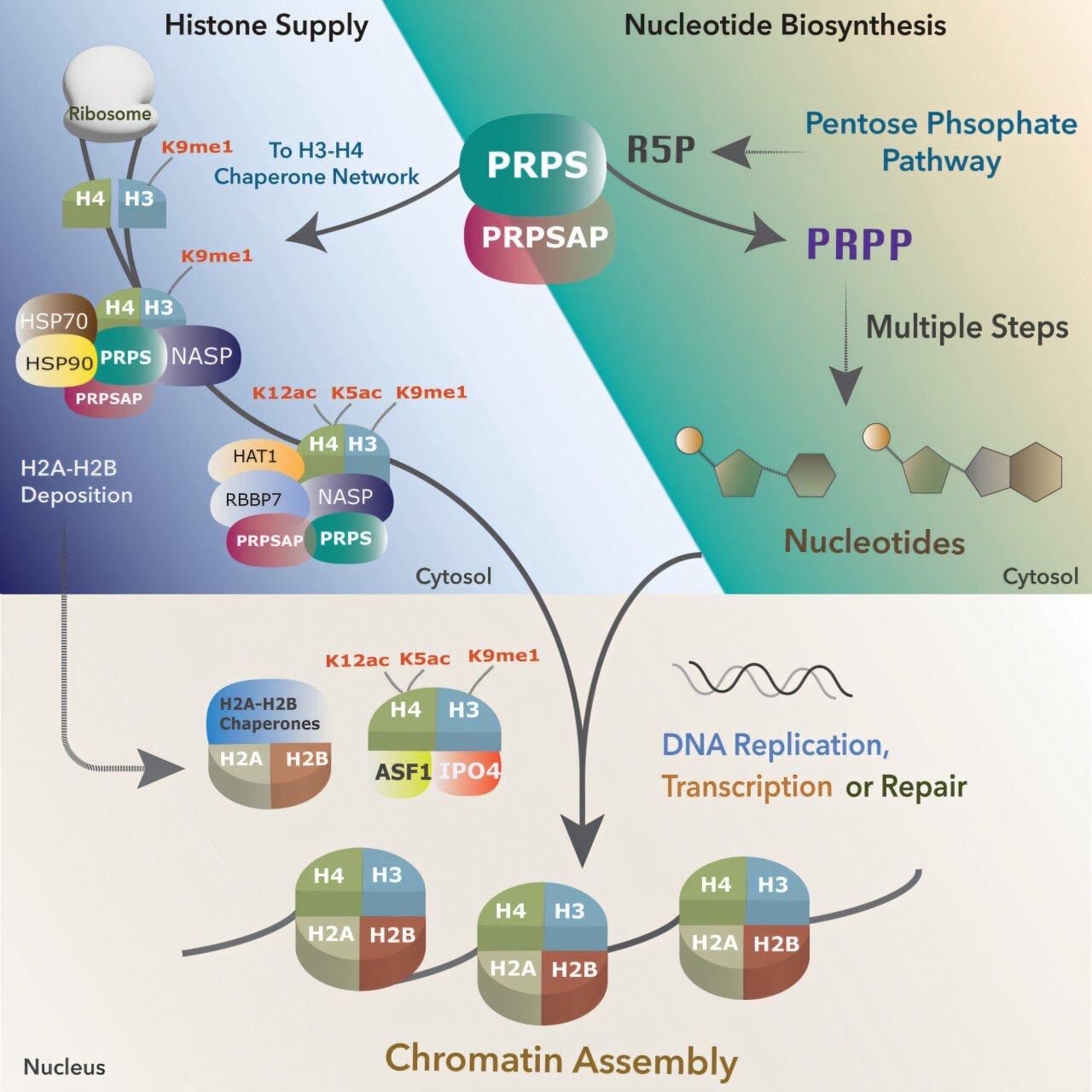
In this study, researchers discovered that PRPS enzymes do more than just support metabolism. When PRPS1 or PRPSAP1 was rapidly depleted, cells showed severe defects in histone maturation and deposition, even when DNA replication itself appeared largely intact. Newly synthesized histones accumulated in the cell but failed to be properly incorporated into chromatin.
This finding revealed a dual function for PRPS enzymes. Alongside their metabolic role, they also help regulate early steps in histone handling, effectively synchronizing nucleotide availability with chromatin assembly.
Separating Metabolism From Chromatin Function
One of the most important aspects of the study was the experimental approach. The researchers used a rapid degron system, allowing them to quickly remove PRPS proteins from cells. This method made it possible to distinguish between the enzymes’ traditional role in nucleotide synthesis and their newly discovered role in histone deposition.
The results showed that histone deposition was acutely sensitive to PRPS complex function and that this sensitivity occurred independently of detectable DNA replication defects. In other words, even when cells still had enough nucleotides to copy DNA, they struggled to assemble chromatin properly if PRPS enzymes were missing.
This clearly demonstrated that PRPS enzymes are directly involved in chromatin assembly, not merely influencing it indirectly through metabolism.
How PRPS Enzymes Support Histone Deposition
The study suggests that PRPS enzymes interact with histone chaperones, the proteins responsible for escorting newly made histones to DNA. By engaging with this chaperone network, PRPS enzymes help ensure that histones are correctly processed and delivered at the right time during replication.
This coordination allows DNA synthesis and chromatin assembly to proceed smoothly and in sync. When this balance is disrupted, histones are not deposited efficiently, leaving DNA vulnerable.
Implications for Genome Stability and Disease
Chromatin assembly plays a central role in protecting genome integrity. When histone supply or deposition is compromised, cells experience increased DNA damage and replication stress. These conditions are closely associated with cancer, neurological disorders, and aging-related diseases.
PRPS mutations have already been linked to several human conditions, including metabolic syndromes and certain cancers. The new findings suggest that these diseases may not arise solely from disrupted nucleotide production. Instead, defects in the newly identified coordination between metabolism and chromatin assembly could also contribute to disease development.
This opens up new research directions, including the possibility that targeting chromatin-related functions of metabolic enzymes could offer novel therapeutic strategies.
A Broader View of Cellular Organization
Beyond PRPS enzymes specifically, the study highlights a broader biological principle. Cellular biosynthetic pathways may be far more interconnected than previously thought. Processes once assumed to operate independently may actually be functionally linked to ensure efficiency and accuracy during cell growth and division.
In the context of DNA replication, this makes intuitive sense. Producing DNA without histones, or histones without DNA, would be wasteful and dangerous. By linking these pathways at the molecular level, cells reduce the risk of imbalance.
Additional Background: Nucleotide Metabolism and Chromatin
Nucleotide metabolism is a highly regulated process that supplies cells with the raw materials needed for DNA and RNA synthesis. PRPS enzymes sit at a critical control point in this pathway, making them essential for cell proliferation.
Chromatin assembly, on the other hand, involves not only histones but also a wide array of chaperones, modifying enzymes, and remodeling complexes. These systems work together to package DNA, regulate gene expression, and maintain epigenetic information.
The discovery that a key metabolic enzyme complex participates directly in chromatin assembly helps bridge these two areas of biology, which have often been studied separately.
What Comes Next
The researchers plan to investigate whether disruptions in this newly identified link contribute directly to disease. Understanding how PRPS enzymes interact with histone chaperones at a molecular level will be an important next step.
More broadly, the study encourages scientists to look for similar dual-purpose roles in other metabolic enzymes. It is increasingly clear that metabolism is not just about energy and building blocks, but also about regulating complex cellular processes like genome maintenance.
By revealing a hidden connection between nucleotide metabolism and chromatin assembly, this work represents a significant step forward in understanding how human cells balance the demands of DNA replication, gene regulation, and long-term genome stability.
Research paper:
https://doi.org/10.1016/j.molcel.2025.11.009