Ancient Bacterial Enzyme Brings Scientists Closer to Sustainable Ethylene Production
Researchers have taken a major step toward producing ethylene, one of the world’s most essential industrial chemicals, in a sustainable way. Ethylene is the key ingredient for making many everyday plastics, from packaging to PVC pipes, but it currently comes almost entirely from fossil fuels through energy-intensive processes. A new study in Nature Catalysis reveals how an ancient bacterial enzyme, known as methylthio-alkane reductase (MAR), could someday replace fossil-fuel-based production with a cleaner, bio-based method.
Discovering the Enzyme That Makes Ethylene
The breakthrough comes from scientists at The Ohio State University, UCLA, and U.S. Department of Energy laboratories. They identified and studied the enzyme MAR, which some bacteria naturally use to extract sulfur from organic molecules. In the process, the bacteria also generate ethylene, the same gas used in modern plastics manufacturing.
Until now, no one had isolated this enzyme from bacteria or determined exactly how it works. The Ohio State research team, led by microbiologist Justin North, succeeded in purifying MAR from the soil bacterium Rhodospirillum rubrum. That single achievement allowed them to finally study MAR’s structure, function, and chemical mechanisms in detail.
This discovery builds on a 2020 study by North’s group that hinted at the presence of ethylene-producing enzymes in bacteria. However, the enzyme’s identity and operation were unclear until now.
How MAR Works Inside Bacteria
MAR’s natural role is in bacterial sulfur metabolism. When sulfate—the typical sulfur source—is scarce, some bacteria switch to harvesting sulfur from volatile organic sulfur compounds (VOSCs), such as methylthio-ethanol (MT-EtOH) or dimethyl sulfide (DMS).
In this process, MAR breaks these compounds apart, releasing methanethiol (CH₃-SH)—a useful sulfur-containing molecule—and small hydrocarbons like ethylene (C₂H₄) and methane (CH₄) as byproducts.
The enzyme’s structure and function turned out to be remarkably similar to nitrogenase, the ancient enzyme that allows bacteria to convert nitrogen gas (N₂) into biologically useful nitrogen. This similarity is more than superficial—both enzymes belong to the same nitrogenase superfamily, and both rely on complex metal clusters made of iron and sulfur for their chemical reactions.
A Closer Look at MAR’s Structure
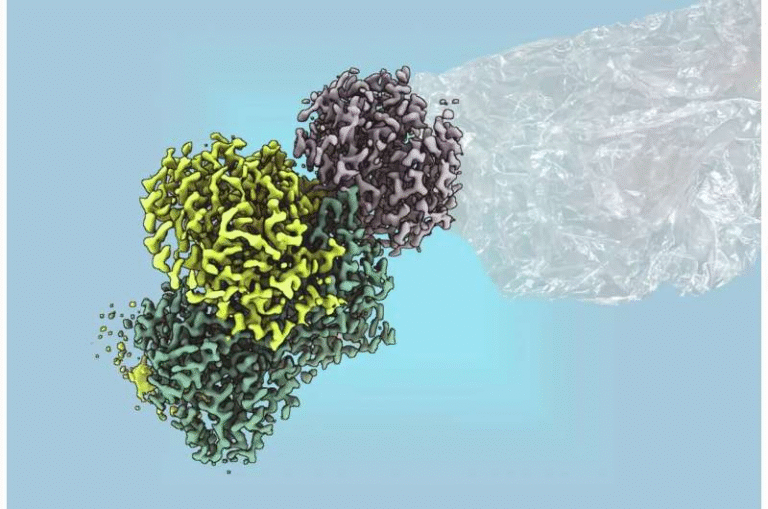
Researchers at Brookhaven National Laboratory and UCLA used cryogenic electron microscopy (cryo-EM) and advanced spectroscopy to uncover how MAR is built and how it works.
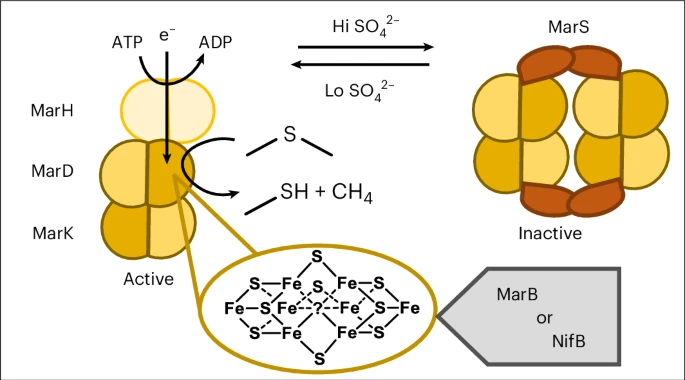
The MAR complex includes multiple subunits:
- MarH, an ATP-dependent reductase that transfers electrons.
- MarD and MarK, which form the catalytic core responsible for the reaction itself.
- MarS, a regulatory component that can suppress MAR activity when sulfur becomes abundant.
The metal cofactors within the enzyme—called mar1 and mar2 clusters—are chemically similar to the P-cluster and M-cluster in nitrogenase, but not identical. They provide MAR with the ability to manage electrons and perform the difficult chemistry of breaking carbon–sulfur bonds and forming ethylene.
This enzyme only functions under anaerobic (oxygen-free) conditions, and it requires ATP and electron donors to drive its reactions—once again paralleling nitrogenase chemistry.
When scientists added sulfate back into the bacterial system, MAR activity shut down. The MarS component binds to the catalytic core and prevents further reaction, a built-in control mechanism that helps bacteria conserve energy when sulfur is plentiful.
The First Isolation and Structural Revelation
Isolating MAR was a significant technical challenge. The team used synthetic biology tools provided by the Joint Genome Institute (JGI), where scientists like Yasuo Yoshikuni engineered multiple versions of MAR genes for testing. When expressed in Rhodospirillum rubrum, these genes produced enough MAR for purification—something no research group had achieved before.
With purified MAR in hand, the team could finally explore its spectroscopic signatures and electron flow pathways. Detailed experiments by Hannah Shafaat’s group at UCLA showed how MAR’s metal clusters handle electrons during ethylene production. The results confirmed that MAR is, in a sense, an evolutionary cousin of nitrogenase, performing a different job but using a similar chemical playbook.
Why This Discovery Matters
This finding opens a new route to bio-based ethylene production. Today, ethylene is made by steam-cracking fossil hydrocarbons, a process that consumes enormous amounts of energy and releases large amounts of carbon dioxide. If scientists can adapt MAR or a modified version of it for industrial use, ethylene could be produced from renewable feedstocks—possibly from waste organic matter or engineered microbes.
The researchers envision a future where MAR-powered bacteria could produce ethylene in fermentation tanks instead of factories burning fossil fuels. However, the work is still at an early stage.
Challenges to Overcome
Although this discovery is promising, several challenges remain before bio-ethylene becomes a practical reality:
- Oxygen sensitivity: MAR only works in oxygen-free environments. Industrial conditions would need to protect the enzyme or engineer oxygen-resistant variants.
- Reaction speed: Like nitrogenase, MAR operates relatively slowly compared to synthetic catalysts. Improving its turnover rate is essential for commercial use.
- Substrate cost: MAR uses volatile sulfur compounds as input. To scale up, scientists must find inexpensive and renewable substrate sources.
- Host engineering: Successfully inserting MAR into a production microbe like E. coli or yeast requires careful tuning of electron transfer systems and cofactor assembly pathways.
- Economic competition: Petrochemical ethylene is currently cheap to make. Biotechnological production must become cost-competitive through innovation and scaling.
Despite these hurdles, this study provides the blueprint scientists need to start optimizing MAR for industrial chemistry.
The Bigger Picture: What Are Nitrogenase-Like Enzymes?
The nitrogenase superfamily includes some of biology’s most fascinating and complex enzymes. They all share a core structure that holds elaborate metal–sulfur clusters capable of handling multi-electron reactions.
Classic nitrogenases help microbes “fix” nitrogen gas into ammonia, fueling the global nitrogen cycle and enabling plant life. The discovery of MAR adds another dimension to this enzyme family—it shows that similar machinery can also handle sulfur chemistry and hydrocarbon production.
In evolutionary terms, MAR may be a more ancient relative of nitrogenase. It may have evolved in early anaerobic microbes that needed to extract sulfur from their environment long before oxygen became abundant on Earth. Understanding MAR not only helps scientists design sustainable chemical processes but also offers clues about how metabolism evolved billions of years ago.
Looking Ahead
Now that MAR’s structure and function are known, the next step is enzyme engineering. Scientists can modify the enzyme’s active sites, metal clusters, and protein interfaces to boost performance and stability. The ultimate goal is to create a supercharged MAR that produces ethylene quickly and reliably under realistic conditions.
Such an achievement could revolutionize the plastics industry. Ethylene is the backbone of polyethylene and many other polymers used globally. A biological method to make it could drastically reduce greenhouse gas emissions associated with plastic production.
The researchers behind this study see their discovery as an important milestone on that path. While practical applications are still years away, MAR demonstrates nature’s hidden ability to perform industrially valuable chemistry with elegance and precision.
Summary
This study uncovers how a long-hidden bacterial enzyme, methylthio-alkane reductase (MAR), can break sulfur-containing molecules apart to create ethylene. By solving its structure and understanding how its unique iron–sulfur clusters work, scientists have opened the door to a sustainable method of making one of the world’s most important chemicals.
The findings combine microbiology, structural biology, and green chemistry, offering a new vision for producing materials that shape modern life—without relying on fossil fuels.
Research Reference:
Architecture, catalysis and regulation of methylthio-alkane reductase for bacterial sulfur acquisition from volatile organic compounds – Nature Catalysis (2025)