Catalyst Behavior That Could Cut Emissions and Stabilize the Supply of Everyday Materials Revealed
A research team led by Rice University has uncovered new details about how industrial catalysts behave during the production of vinyl acetate monomer (VAM), a chemical that quietly supports a massive portion of modern manufacturing. VAM is a core building block for adhesives, paints, coatings, packaging materials, textiles, and construction products, making its efficient and stable production critically important for both industry and everyday life.
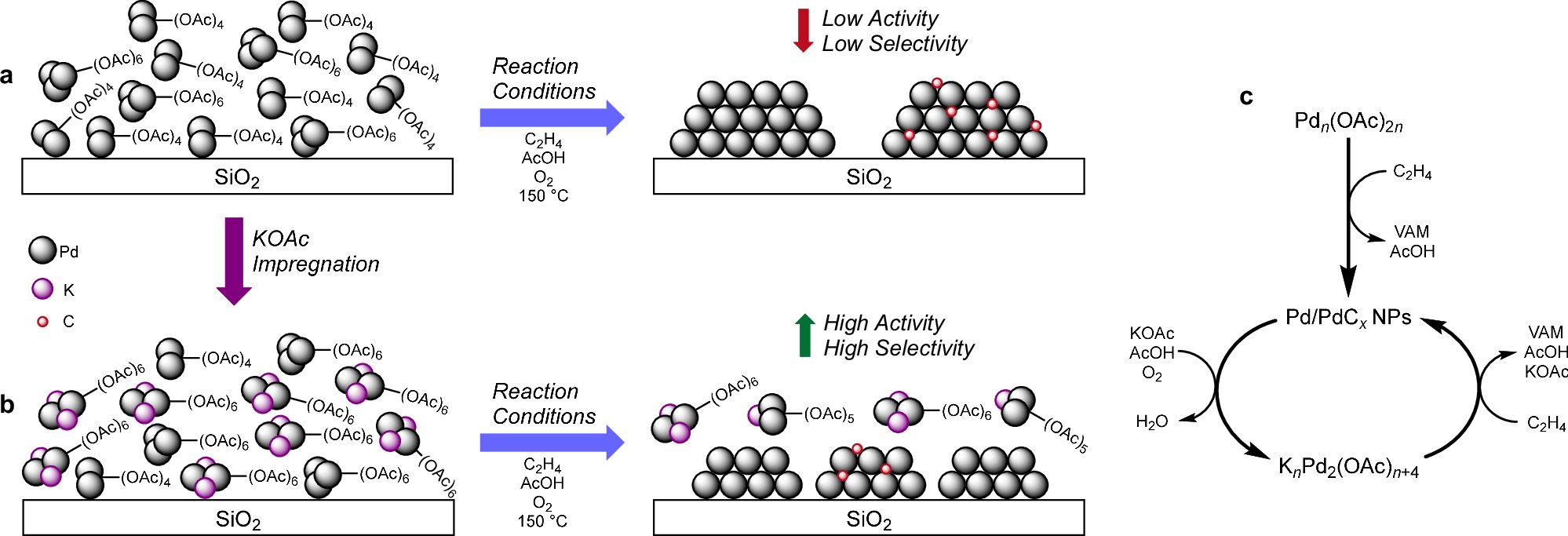
At the heart of this study is a closer look at how molecular-scale palladium-acetate structures behave under real industrial reaction conditions. These tiny structures, known as palladium-acetate dimers and trimers, were once thought to be inactive byproducts or early signs that a catalyst was degrading. The new research shows the opposite: these molecular species are actively involved in controlling catalyst performance, energy use, and emissions.
Why Vinyl Acetate Monomer Matters So Much
Vinyl acetate monomer is produced on a global scale, with millions of tons manufactured every year. It is created by reacting ethylene, oxygen, and acetic acid over a palladium–gold catalyst that is promoted with potassium acetate. Even small inefficiencies in this process can lead to higher energy consumption, more waste, and increased carbon dioxide emissions.
Because VAM sits at the base of so many supply chains, improvements in how it is produced can have wide-reaching environmental and economic impacts. More efficient catalysts mean less raw material wasted, fewer greenhouse gases released, and more predictable production for industries that depend on VAM-derived products.
A Closer Look at Palladium-Acetate Species
The researchers focused on simplified palladium-acetate model catalysts to understand what happens at the molecular level during VAM synthesis. Using a combination of advanced X-ray techniques, spectroscopy, electron microscopy, and computational modeling, they tracked how palladium-acetate trimers and dimers evolve once the reaction starts.
What they found was striking. Instead of being inert or harmful, these molecular species participate in a dynamic redox cycle. This cycle controls how palladium transitions from molecular complexes into metallic palladium nanoparticles, which are the true workhorses of the catalyst.
When these nanoparticles remain small and evenly dispersed, the catalyst becomes both more active and more selective. That selectivity is crucial because it reduces unwanted side reactions that convert valuable feedstocks into carbon dioxide instead of useful product.
The Role of Potassium Acetate
One of the most important findings involves potassium acetate, a promoter already used in industrial VAM catalysts. The study shows that potassium acetate plays a critical role in stabilizing specific palladium-acetate dimers. By doing so, it influences how quickly and in what way these molecular species convert into metallic palladium nanoparticles.
With potassium acetate present, the catalyst tends to form smaller, well-dispersed palladium particles. Without it, palladium species are more likely to collapse into larger palladium or palladium carbide (PdCx) nanoparticles, which are less efficient and less selective.
This insight gives chemists and engineers a powerful new lever to adjust catalyst behavior. By fine-tuning how these molecular species form and transform, it becomes possible to design catalysts that operate at lower temperatures, use less energy, and produce less waste.
Rethinking Catalyst Deactivation
For years, palladium-acetate trimers and dimers were often seen as warning signs that a catalyst was on its way to deactivation. This study reframes that assumption entirely. Instead of being passive or harmful, these species act as active regulators of nanoparticle growth.
In practical terms, they serve as indicators of catalyst health. Their presence and behavior can signal whether a catalyst is likely to remain stable or drift toward less efficient forms. This opens the door to smarter catalyst monitoring and longer catalyst lifetimes in industrial reactors.
Industry Collaboration and Real-World Impact
The research was conducted in collaboration with Celanese Corporation, one of the world’s leading producers of vinyl acetate monomer, as well as partners at Purdue University and Oak Ridge National Laboratory. This close industry-academia partnership ensured that the experiments were designed around realistic reaction conditions, not just idealized lab scenarios.
For large-scale producers, the implications are significant. Improved catalyst stability means fewer shutdowns, lower maintenance costs, and more consistent product quality. From a sustainability perspective, higher selectivity directly translates into lower carbon emissions, since fewer molecules are burned off as carbon dioxide.
How Advanced Tools Made This Possible
Understanding catalyst behavior at this level required a powerful combination of experimental and computational tools. The team used operando techniques, which allow scientists to observe catalysts while they are actively working. Transmission electron microscopy provided direct images of how nanoparticles form and evolve, while computational models helped explain why certain molecular species stabilize specific structures.
This integrated approach allowed the researchers to connect molecular-scale behavior directly to industrial performance metrics like efficiency, selectivity, and emissions.
What This Means for Cleaner Chemical Manufacturing
Although the study focuses on VAM, its implications extend far beyond a single chemical. Many industrial processes rely on heterogeneous catalysts that undergo similar molecular-to-nanoparticle transformations. The idea that small molecular species can actively guide nanoparticle formation could influence catalyst design across the chemical industry.
Better catalysts mean lower energy demand, reduced greenhouse gas emissions, and more resilient supply chains. In industries where margins are tight and environmental pressure is increasing, these incremental improvements can add up to substantial gains.
A Broader Perspective on Catalysts and Sustainability
Catalysts are often invisible to the public, but they play a central role in nearly every modern material. From plastics and pharmaceuticals to fuels and fertilizers, catalysts determine how efficiently raw materials are transformed into useful products. Research like this highlights how deep molecular understanding can unlock practical solutions to sustainability challenges.
As global demand for materials continues to grow, the ability to produce them more cleanly and reliably will only become more important. Insights into palladium-acetate behavior offer a clear example of how fundamental science can directly support climate goals, industrial efficiency, and economic stability at the same time.
Research paper:
https://www.nature.com/articles/s41467-025-66820-7