Creating Psychedelic-Like Molecules by Shining Light on Life’s Basic Building Blocks
Researchers at the University of California, Davis have uncovered a striking new way to design potential brain-targeting drugs—by using light to transform amino acids, the most fundamental building blocks of life, into molecules that behave like psychedelics but do not cause hallucinations. The discovery opens up a promising new direction for psychiatric drug development, especially for conditions such as depression, PTSD, and substance-use disorders.
The work comes from scientists in the UC Davis Department of Chemistry and the Institute for Psychedelics and Neurotherapeutics (IPN) and was recently published in the Journal of the American Chemical Society. What makes this research stand out is not just the medical potential, but the chemistry itself: a simple, light-driven process that creates entirely new drug-like molecules from materials already found in nature.
Turning amino acids into serotonin-active molecules
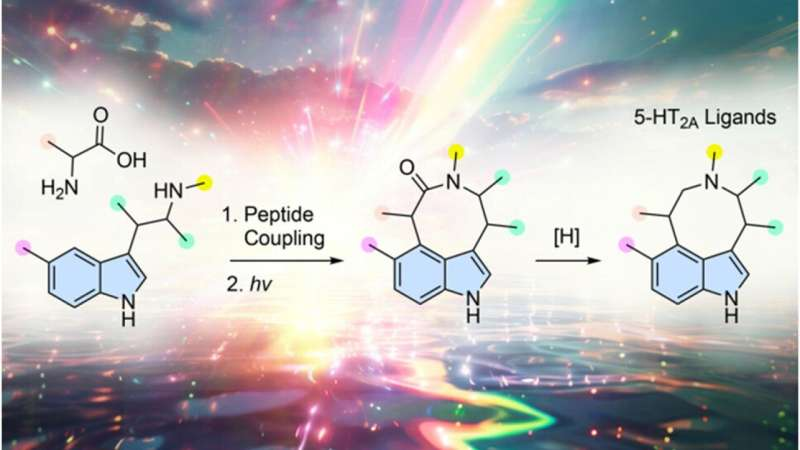
At the center of the study is a clever use of photochemistry, which involves driving chemical reactions using light. The research team started with tryptamine, a naturally occurring compound derived from the essential amino acid tryptophan. Tryptamine is already well known in neuroscience because it forms the backbone of many classic psychedelics.
The scientists chemically linked tryptamine with a variety of other amino acids and then exposed these hybrids to ultraviolet light. Under irradiation, the molecules underwent structural rearrangements, forming entirely new compounds that do not exist in nature.
These new molecules share structural features with psychedelics but are chemically distinct, forming what researchers describe as a brand-new therapeutic scaffold. In medicinal chemistry, discovering a completely new scaffold is rare, especially in the psychedelic research space, where most drugs are variations of known compounds like psilocybin or LSD.
Why serotonin 5-HT2A receptors matter
The newly created compounds were designed to interact with the serotonin 5-HT2A receptor, a key protein in the brain that plays a central role in perception, mood, and cognition. This receptor is also the main target of classical psychedelics, and its activation has been linked to increased neural plasticity, meaning the brain’s ability to adapt and rewire itself.
Activation of the 5-HT2A receptor has drawn intense interest from researchers because it may help explain why psychedelic-inspired treatments show promise for mental health conditions that are otherwise difficult to treat. However, traditional psychedelics also produce hallucinations, which complicates their clinical use.
The UC Davis team wanted to know whether it was possible to separate the therapeutic benefits from the hallucinogenic effects.
Screening and selecting promising candidates
To answer that question, the researchers created a library of around 100 newly formed molecules. Using computer simulations, they examined how strongly each compound might bind to the 5-HT2A receptor.
From this large group, five candidates were selected for detailed laboratory testing. These compounds were examined for both potency and efficacy, meaning how strongly and how effectively they activate the receptor.
The results were impressive. The selected compounds showed efficacies ranging from 61% to 93%, with the highest-performing molecule acting as a full agonist. A full agonist is capable of producing the maximum possible response from a receptor.
That standout molecule was labeled D5.
A surprising result in animal testing
Based on everything known about serotonin biology, the researchers expected D5 to behave like a classic psychedelic. In animal studies, psychedelics reliably trigger a behavior known as the head-twitch response in mice, which is widely used as a proxy for hallucinogenic activity.
But when D5 was administered to mouse models, the expected behavior never appeared.
Despite fully activating the same receptor that produces hallucinations in response to psychedelics, D5 did not induce head-twitch responses. Instead, the compound appeared to suppress psychedelic-like behaviors.
This unexpected result suggests that activating the 5-HT2A receptor alone is not enough to cause hallucinations. The way a molecule binds to the receptor, the signaling pathways it triggers, and how it interacts with other serotonin receptors all seem to matter.
Understanding why hallucinations don’t occur
Laboratory and computational studies showed that these light-generated molecules can activate serotonin signaling pathways linked to both brain plasticity and hallucinogenic effects. Yet in live animals, the hallucinogenic response was absent.
The researchers now believe that other serotonin receptors or signaling mechanisms may be modulating or suppressing hallucination-related activity. Future studies will focus on identifying exactly how compounds like D5 engage the serotonin system differently from traditional psychedelics.
Understanding this distinction could be crucial for designing next-generation psychiatric medications that deliver therapeutic benefits without profound perceptual changes.
A greener and more flexible drug discovery approach
Beyond neuroscience, the chemistry itself is notable for being efficient and environmentally friendly. Traditional drug discovery often involves multi-step synthesis processes that rely on harsh chemicals and expensive reagents. In contrast, this method uses simple starting materials and light as the driving force, making it both scalable and adaptable.
By swapping different amino acids into the starting materials, researchers can rapidly generate a wide variety of related compounds. This creates a modular platform for discovering new serotonin-active drugs, potentially speeding up the development of future treatments.
What this means for mental health research
This work fits into a growing scientific effort to develop non-hallucinogenic psychoplastogens—compounds that promote brain plasticity without causing altered states of consciousness. Such drugs could offer many of the benefits associated with psychedelic therapy while being easier to prescribe, regulate, and integrate into standard medical practice.
Conditions like major depressive disorder, post-traumatic stress disorder, and addiction are often resistant to existing treatments. Drugs that safely enhance neural flexibility could help patients break out of harmful thought patterns without the risks associated with hallucinogenic experiences.
Looking ahead
The UC Davis team emphasizes that this is an early-stage discovery, but one with significant implications. The identification of a new therapeutic scaffold, combined with a straightforward synthesis method and surprising biological behavior, provides a strong foundation for future research.
Follow-up studies will explore how these molecules behave across different serotonin receptors, how long their effects last, and whether they can be optimized for safety and effectiveness in humans.
What began as a chemistry experiment shining ultraviolet light on amino acids may ultimately reshape how scientists think about psychedelic-inspired medicine—proving that profound biological effects can emerge from surprisingly simple ingredients.
Research paper:
https://doi.org/10.1021/jacs.5c19817