Molecular Reshuffle Solves an 80-Year-Old Problem in Controlling Chirality
Researchers at the University of St Andrews have achieved something chemists have struggled with for decades: they have uncovered a hidden molecular step that finally explains how to control chirality in a famously unpredictable chemical reaction. The discovery sheds light on an 80-year-old puzzle in organic chemistry and could significantly improve how medicines and advanced materials are made.
The findings were published on 6 January 2026 in the journal Nature Chemistry, marking a major step forward in understanding how complex molecules can be built with precision. The research was carried out by scientists from the School of Chemistry at the University of St Andrews, working closely with collaborators at the University of Bath.
At the center of the study is a reaction known as the [1,2]-Wittig rearrangement, a transformation first discovered more than eight decades ago. While chemists have long known how this rearrangement works at a basic level, controlling its outcome—especially when it comes to molecular handedness—has proven extremely difficult. Until now, the reaction was widely viewed as too erratic to be useful for modern, selective chemical synthesis.
Why Chirality Matters in Chemistry
Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image, much like left and right hands. These mirror-image forms are called enantiomers, and despite having the same chemical formula, they can behave very differently in biological systems.
This difference is especially important in pharmaceutical chemistry. In many drugs, only one enantiomer delivers the intended therapeutic effect, while the other may be inactive or even harmful. Because of this, chemists are constantly searching for reliable ways to produce molecules in one specific handed form rather than a mixture of both.
Controlling chirality is one of the biggest challenges in synthetic chemistry, and reactions that rearrange atoms within a molecule are often the hardest to tame. The [1,2]-Wittig rearrangement has been a textbook example of this problem.
The Longstanding Problem with the [1,2]-Wittig Rearrangement
The [1,2]-Wittig rearrangement involves the movement of atoms within a molecule, typically converting ethers into alcohols through a rearrangement process. While the reaction is useful in theory, it has long been considered stereochemically uncontrollable. In other words, chemists could not reliably predict or dictate which enantiomer would be formed.
Because of this unpredictability, the reaction was largely avoided in situations where precise control of molecular structure was required, such as in drug development or the synthesis of complex functional materials. For decades, it remained a fascinating but frustrating chemical transformation.
The Discovery of a Hidden Molecular Reshuffle
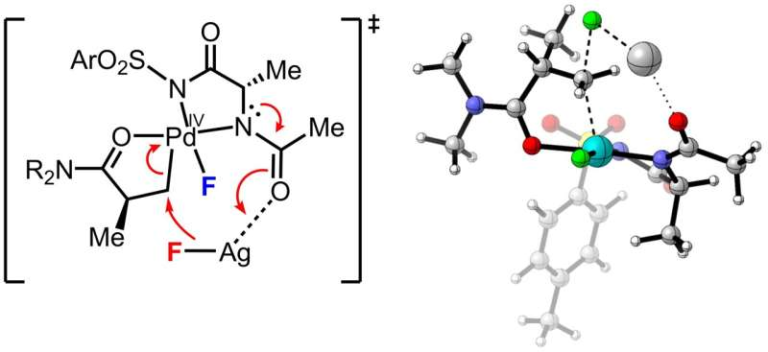
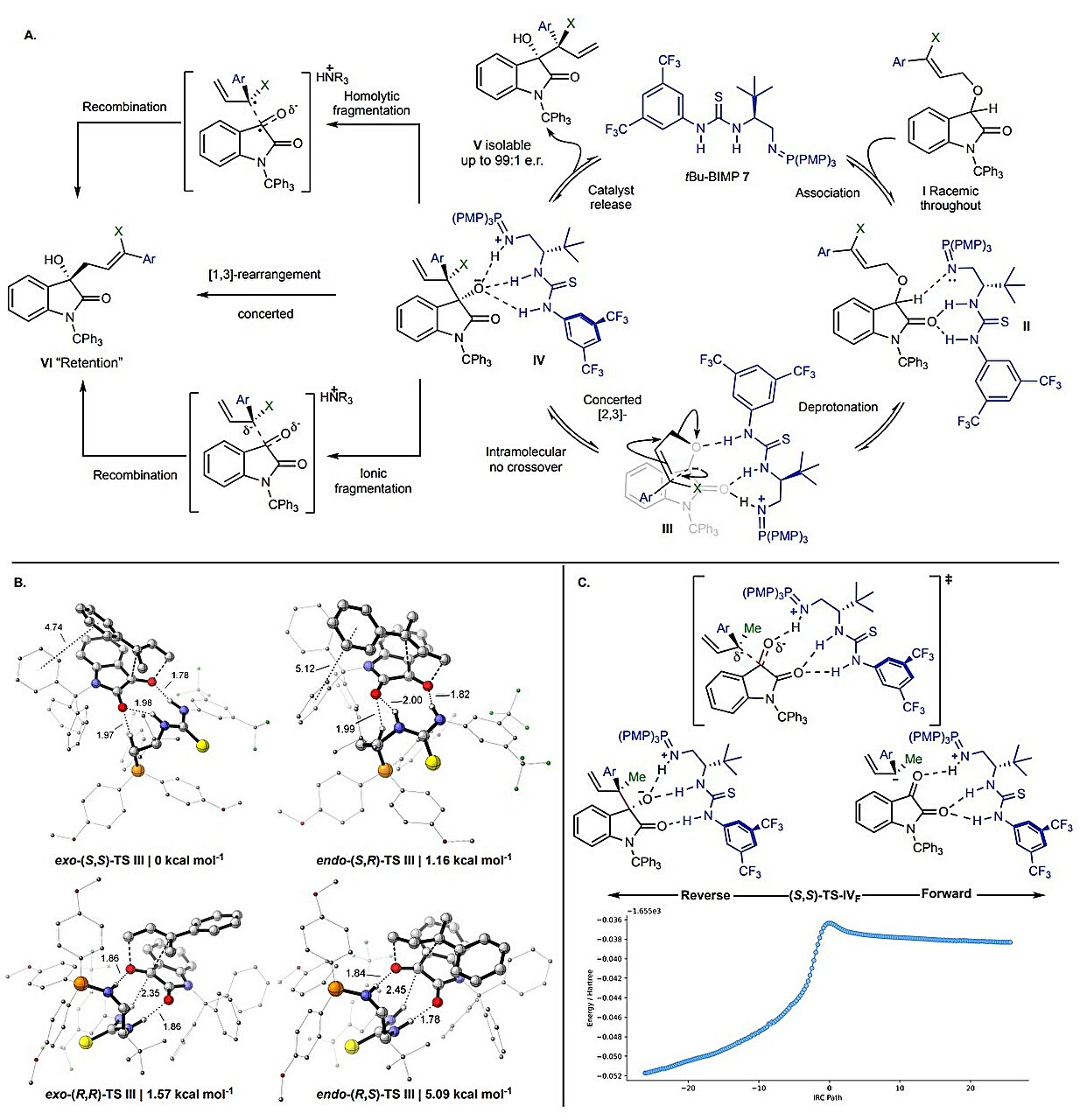
The new study changes this understanding completely. By combining laboratory experiments with advanced quantum chemistry calculations, the research team uncovered a previously unknown step in the reaction mechanism—a molecular “reshuffle” that plays a crucial role in preserving chirality.
The scientists found that the reaction does not proceed in a single chaotic leap, as previously assumed. Instead, a catalyst first guides the molecule through an initial asymmetric rearrangement, which sets the molecule’s handedness early in the process. This is followed by a previously unrecognized reshuffling of atoms that maintains that handedness rather than scrambling it.
This insight explains why the reaction can, in fact, be controlled under the right conditions. The reshuffle step effectively locks in the chirality established by the catalyst, making the overall process enantioselective—a quality that chemists had long believed was impossible for this reaction.
A Shift in How Chemists Understand Rearrangement Reactions
This discovery represents a fundamental shift in how chemists think about rearrangement reactions and stereochemistry. Mechanistic pathways that were once dismissed as unreliable or unusable may now be revisited with fresh eyes.
The work also demonstrates the power of combining experimental chemistry with computational modeling. Without detailed quantum chemical calculations, the subtle reshuffle step might have remained hidden, continuing to confuse chemists for generations.
By clearly mapping out the full catalytic cycle, the researchers have provided a blueprint that other scientists can use to design new asymmetric transformations based on similar principles.
Implications for Drug Manufacturing and Materials Science
One of the most exciting aspects of this discovery is its potential impact on real-world chemical manufacturing. The ability to control chirality in reactions that were once considered uncontrollable could lead to faster, cleaner, and more selective synthetic routes.
In pharmaceutical production, this could mean fewer steps, less chemical waste, and greater confidence that only the desired enantiomer is produced. In materials science, precise control over molecular structure can influence properties such as optical activity, conductivity, and mechanical strength.
Because the [1,2]-Wittig rearrangement involves relatively simple starting materials, making it enantioselective opens the door to its use in a wide range of applications that were previously off-limits.
Broader Context: Why This Discovery Matters Beyond One Reaction
Beyond the specifics of the Wittig rearrangement, this research highlights a broader lesson in chemistry: long-standing assumptions can still be wrong. Reactions labeled as “uncontrollable” may simply be misunderstood.
The discovery of a molecular reshuffle suggests that other classic reactions might also hide overlooked steps that, once understood, could be harnessed for precise chemical control. This has implications for reaction design, catalyst development, and mechanistic education in chemistry.
It also reinforces the idea that progress often comes not from inventing entirely new reactions, but from deeply understanding old ones.
Key Details of the Research
The study is titled “The catalytic enantioselective [1,2]-Wittig rearrangement cascade of allylic ethers” and appears in Nature Chemistry (2026). It focuses on allylic ethers and demonstrates how a carefully designed catalyst and mechanistic insight can overcome decades of uncertainty.
The collaboration between the University of St Andrews and the University of Bath played a crucial role, combining expertise in experimental organic chemistry and computational analysis.
Looking Ahead
With this breakthrough, chemists now have a new framework for thinking about stereochemical control in rearrangement reactions. The discovery is likely to inspire further research into hidden mechanistic steps, not only in Wittig-type reactions but across many areas of synthetic chemistry.
As the demand for highly selective and sustainable chemical processes continues to grow, insights like this could shape the next generation of chemical innovation.
Research paper:
https://www.nature.com/articles/10.1038/s41557-025-02022-4