Pulsed Electrolysis Could Turn Airborne Nitrogen Into Sustainable Fertilizer

Nitrogen-based fertilizers like ammonia and urea are essential for feeding the planet. They keep farmlands fertile and crops productive, but their traditional production methods are anything but clean. The world still depends heavily on the Haber–Bosch process, a century-old industrial method that fuses nitrogen and hydrogen under extremely high temperatures—around 400 to 500 degrees Celsius—and intense pressure. While it revolutionized agriculture in the early 20th century, it also created a huge energy and environmental burden.
This process alone accounts for about 1% of global energy consumption and emits significant greenhouse gases. Worse still, the fertilizers it produces often wash off fields into rivers and oceans, causing nitrogen pollution and algal blooms that damage aquatic ecosystems. The situation is compounded by nitrous oxide (N₂O), a potent byproduct of fertilizer production and use, which has nearly 300 times the global warming potential of carbon dioxide.
Now, a team of researchers from Johannes Gutenberg University Mainz (JGU) in Germany and the Harbin Institute of Technology in Shenzhen, China believes there’s a cleaner way forward. Their latest study, published in Angewandte Chemie International Edition, highlights how pulsed electrolysis could transform the way we harvest nitrogen—offering a greener, more flexible route to producing fertilizers from the air and water itself.
How Pulsed Electrolysis Works
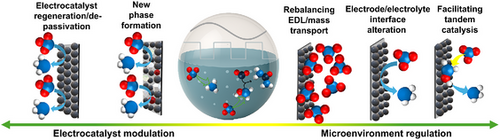
In a nutshell, pulsed electrolysis is an upgraded form of electrochemical nitrogen conversion. Instead of maintaining a constant electrical voltage, this method varies the voltage and current in timed pulses. That rhythmic switching—between active and resting phases—creates a dynamic environment at the electrode surface.
The process starts with nitrogen-rich water, containing compounds such as nitrates (NO₃⁻) or nitrites (NO₂⁻). When electricity is applied, these compounds can be reduced to ammonia (NH₃) or even urea, all at room temperature and under normal atmospheric pressure.
By pulsing the current rather than keeping it steady, researchers found that they could boost efficiency and selectivity—meaning more of the input energy goes into creating useful nitrogen products and less is wasted on unwanted reactions like hydrogen gas evolution.
This technique also works naturally with intermittent renewable power sources like solar and wind. Because those energy sources fluctuate, a pulsed system can easily sync with variable input, unlike traditional chemical plants that require constant, stable power.
Why This Is a Big Deal
The implications go far beyond laboratory curiosity. If scaled successfully, pulsed electrolysis could rewrite the nitrogen cycle for agriculture and industry.
Right now, large centralized ammonia plants rely on fossil fuels—usually natural gas—to extract hydrogen, releasing carbon dioxide in the process. By contrast, pulsed electrolysis can use electricity from renewable sources and water as the hydrogen source. That means zero direct CO₂ emissions, lower energy requirements, and potentially smaller, localized production systems.
In the future, farms might even be able to produce their own fertilizers on-site, powered by wind or solar panels. This decentralization could cut down transport emissions and allow farmers to adjust fertilizer output based on immediate demand.
Moreover, because pulsed electrolysis can use waste nitrogen compounds—like nitrates found in contaminated water bodies—it offers a double environmental benefit: cleaning up nitrogen pollution while producing valuable fertilizer.
What the Researchers Found
Dr. Dandan Gao, a chemist at JGU, and her collaborators compiled and analyzed all available studies on nitrogen reduction through pulsed electrolysis. The review identifies how this method has evolved and where it stands today.
The researchers discovered that pulsing the electrical potential changes several key aspects of the reaction environment:
- Surface activity of the catalyst: During each pulse, the surface atoms of the electrode can restructure or reset, preventing degradation and improving the long-term stability of the reaction.
- Local pH control: Pulses help maintain a balanced environment near the electrode surface. Too alkaline, and the reaction slows; too acidic, and side reactions take over. The rhythmic electrical switching keeps conditions optimal.
- Reduction of competing reactions: Pulsing helps suppress the hydrogen evolution reaction (HER), a common side process that steals energy away from nitrogen reduction.
- Efficient diffusion: When the current stops momentarily, the surrounding ions and molecules have time to redistribute, refreshing the system before the next pulse begins.
Their analysis showed that, under pulsed conditions, yields and Faradaic efficiencies (a measure of how efficiently electrons are used to make the desired product) were significantly improved over conventional steady-state electrolysis.
This kind of control over the electrode microenvironment makes pulsed electrolysis not just an incremental improvement, but a possible game-changer in electrochemical fertilizer production.
From Lab Concept to Real-World Application
Although the concept shows enormous promise, the research is still in its early stages. The team stresses that much work remains before pulsed electrolysis becomes an industrial reality.
Among the challenges are:
- Scaling up the process: Laboratory setups can produce milligrams of ammonia per hour, but industrial-scale production would require thousands of times that output.
- Electrode stability: Constant voltage switching can wear down electrode materials. Researchers are exploring durable catalysts, including metal alloys and nanostructured surfaces, that can withstand repeated cycles.
- Product purification: After ammonia and urea are formed, they must be efficiently separated and concentrated—a nontrivial task for large systems.
- Energy and cost efficiency: While pulsed electrolysis can run on renewable electricity, its overall cost and energy use must still be competitive with existing methods.
- Mechanistic understanding: Scientists are still uncovering the detailed chemistry behind pulsed electrolysis. Exactly how the timing, voltage range, and frequency influence different intermediates remains an active area of investigation.
Despite these hurdles, Gao and her co-authors believe the technology could, in time, provide a roadmap toward a cleaner nitrogen economy—one aligned with sustainable energy production and environmental protection.
Why Nitrogen Fixation Matters So Much
To understand the significance of this breakthrough, it helps to recall why nitrogen fixation is such a big deal.
Nitrogen gas (N₂) makes up about 78% of Earth’s atmosphere, but it’s incredibly stable. Plants and animals can’t use it directly. They need reactive nitrogen compounds—like ammonia, nitrates, or nitrites—to build proteins and DNA.
For most of human history, this reactive nitrogen came from natural processes: lightning, soil bacteria, and animal waste. That changed in 1909, when Fritz Haber and Carl Bosch developed a way to artificially fix nitrogen using high heat and pressure. The Haber–Bosch process made fertilizers cheap and abundant, fueling the Green Revolution and supporting billions of lives.
But the cost has been steep: massive energy use, reliance on fossil fuels, and large-scale nitrogen pollution. If pulsed electrolysis can do the same job under mild conditions, powered by renewable energy, it could represent a second revolution in fertilizer production—this time one that’s climate-friendly.
The Bigger Picture
The concept of electrochemical nitrogen conversion ties into a growing effort to make industrial chemistry more sustainable. Similar electrochemical approaches are being tested for carbon dioxide reduction (turning CO₂ into fuels or plastics) and water splitting (producing hydrogen).
These technologies share a common vision: replacing fossil-fuel-driven chemical plants with renewable-powered electrochemical systems. Pulsed electrolysis is particularly well-suited to this shift because its pulsed operation mirrors the natural variability of renewable electricity.
The ultimate goal is a circular nitrogen economy—one where waste nitrogen is captured and reused, rather than released into the environment. That would mean cleaner rivers, less nitrous oxide in the atmosphere, and fertilizer production that keeps pace with sustainability goals.
Looking Ahead
Dr. Gao and her team hope that by summarizing the field’s progress and identifying open questions, they can inspire further innovation. They envision a future where pulsed electrolysis systems help bridge chemistry and renewable energy, redefining how humanity manages one of its most essential elements.
It’s not an overstatement to say that nitrogen is life itself. From the protein in our food to the DNA in our cells, it’s at the core of biological existence. Finding cleaner ways to harness it could be one of the defining scientific challenges—and triumphs—of this century.
Research Reference:
Reductive Nitrogen Species Activation via Pulsed Electrolysis: Recent Advances and Future Prospects, by Kiarash Torabi, Rongji Liu, David Leander Troglauer, Christean Nickel, Guillermo Corea, Tiansheng Bai, Deping Li, Lijie Ci, Bahareh Feizi Mohazzab, and Dandan Gao, published in Angewandte Chemie International Edition (2025).
https://doi.org/10.1002/anie.202516909