A Simple Mechanical Method Shows Promise for Completely Destroying PFAS on Activated Carbon
Researchers at Clarkson University have reported a surprisingly simple and potentially game-changing way to destroy PFAS, the so-called “forever chemicals,” once they have been captured on granular activated carbon. The approach relies on nothing more than stainless steel ball milling equipment, avoiding the need for added chemicals, high temperatures, or solvents. The findings were published in the journal Environmental Science & Technology Letters in 2025 and directly address one of the biggest unresolved challenges in PFAS remediation: what to do with PFAS after they have been removed from water.
PFAS, short for per- and polyfluoroalkyl substances, are a large group of man-made chemicals that have been used for decades in everyday and industrial products. They appear in nonstick cookware, firefighting foams, water-resistant fabrics, food packaging, and many other applications. What makes PFAS useful in products is also what makes them dangerous in the environment—their extremely strong carbon–fluorine bonds, which resist natural breakdown. Because of this persistence, PFAS can accumulate in water supplies, wildlife, and human bodies, leading to growing concerns about long-term health and environmental impacts.
Why Activated Carbon Matters in PFAS Cleanup
One of the most widely used methods for removing PFAS from contaminated water is granular activated carbon (GAC). Activated carbon is highly porous and has a strong ability to adsorb organic contaminants, including many PFAS compounds. Municipal water systems, industrial facilities, and remediation projects often rely on GAC to trap PFAS before the water is released or reused.
However, while activated carbon is effective at capturing PFAS, it does not destroy them. Once the carbon becomes saturated, it turns into a secondary waste problem. Traditionally, options for dealing with PFAS-laden carbon include incineration, landfilling, or regeneration, all of which come with serious drawbacks. Incineration requires high temperatures and can risk releasing harmful byproducts. Landfilling raises concerns about future PFAS leaching. Regeneration processes are often expensive and energy-intensive.
This is the problem the Clarkson University team set out to address: how to safely and effectively destroy PFAS after they have already been removed from water.
The Core Discovery: Additive-Free Ball Milling
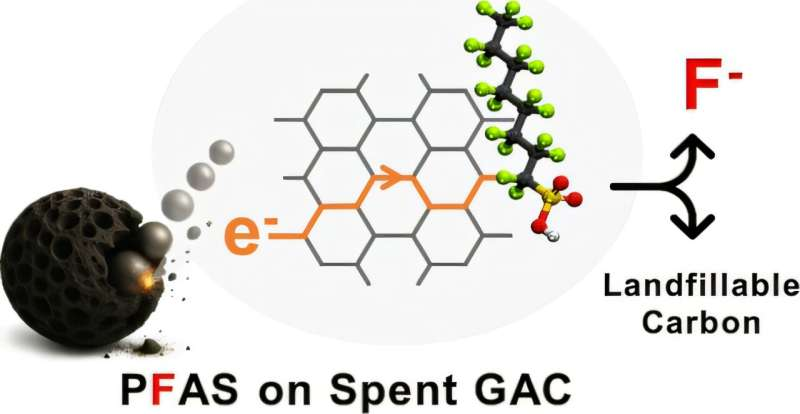
The researchers demonstrated that PFAS adsorbed onto granular activated carbon can be completely destroyed using additive-free ball milling in a stainless steel mill. Ball milling is a mechanical process commonly used in materials science, where rotating steel balls collide with a material, creating intense physical forces.
What makes this approach stand out is what it does not require. There are no added chemicals, no external heat, no solvents, and no specialized catalysts. The process operates at room temperature, making it far simpler than many other PFAS destruction methods currently under investigation.
The key mechanism behind the process involves the generation of triboelectrons. As the stainless steel balls collide during milling, they produce electrons through friction and contact. These electrons then interact with the PFAS molecules adsorbed on the carbon surface, facilitating reactions that break the carbon–fluorine bonds. Once those bonds are broken, the PFAS compounds lose the chemical stability that made them “forever chemicals” in the first place.
Broad Effectiveness Across PFAS Types
An important aspect of the study is that the method worked across multiple types of PFAS, not just a single compound. The researchers tested the process on PFAS commonly found in both laboratory-prepared samples and real-world activated carbon taken from actual use cases. This suggests the method is not limited to idealized conditions but could potentially be applied to real remediation waste.
After milling, the treated carbon samples were subjected to leaching tests designed to simulate landfill conditions. The results showed no detectable release of PFAS, indicating that the chemicals were not merely transformed or redistributed, but effectively destroyed. This finding is especially significant because preventing future PFAS release has been one of the most difficult challenges in managing spent activated carbon.
The study also demonstrated defluorination, meaning fluorine atoms were removed from the PFAS molecules rather than remaining bound in new fluorinated byproducts. This is a critical point, as incomplete PFAS degradation can sometimes result in smaller but still persistent fluorinated compounds.
Why This Matters for PFAS Waste Management
Dealing with PFAS-contaminated materials is a growing global problem. As regulations tighten and testing becomes more widespread, more PFAS-laden waste streams are being identified. Activated carbon, while essential for water treatment, has become one of the most problematic materials to dispose of safely.
This new ball milling approach offers a potential path toward safe disposal of treated carbon, reducing long-term environmental risks. Because the process is mechanical rather than chemical, it could be easier to scale and integrate into existing waste treatment infrastructure. Stainless steel ball mills are already widely used in industry, which could lower barriers to adoption.
While the research does not yet represent a fully commercialized solution, it points toward a future where PFAS removal and destruction can be handled as part of a closed-loop system, rather than shifting contamination from water to solid waste.
Understanding PFAS and Their Risks
PFAS exposure has been linked in scientific studies to a range of potential health effects, including immune system disruption, developmental issues, and increased risks of certain diseases. Because PFAS can persist in the body for long periods, even low-level exposure over time is a concern.
Environmental contamination is equally troubling. PFAS have been detected in rivers, groundwater, soil, and remote ecosystems, far from their original sources. Their ability to travel long distances and resist degradation has earned them the label “forever chemicals,” a term that reflects both their chemistry and the difficulty of dealing with them once released.
How This Fits into the Bigger Picture of PFAS Research
Many current PFAS destruction technologies rely on extreme conditions, such as very high temperatures, plasma systems, or advanced electrochemical treatments. While effective in some cases, these methods can be costly, energy-intensive, and difficult to deploy at scale.
The Clarkson University study stands out because it demonstrates that mechanochemistry alone—using physical forces and electron generation—can achieve meaningful PFAS destruction. This opens the door to further research into low-energy, low-emission treatment methods that could complement existing PFAS removal strategies.
Future work will likely focus on optimizing milling conditions, understanding long-term scalability, and evaluating the economics of deploying this method in real-world waste treatment facilities.
A Practical Step Toward Solving a Persistent Problem
While PFAS contamination remains a complex and evolving challenge, this research offers a rare example of a solution that is both technically elegant and practically grounded. By focusing on what happens after PFAS are removed from water, the study tackles a problem that has often been overlooked.
If further development confirms its scalability and cost-effectiveness, additive-free ball milling could become an important tool in the broader effort to finally deal with PFAS in a way that is safe, sustainable, and genuinely permanent.
Research paper: https://doi.org/10.1021/acs.estlett.5c00976