How Rapidly Transforming Diatom Skeletons Are Quietly Reshaping Ocean Chemistry and Climate
The ocean is full of hidden chemical processes that most of us never hear about, and one of the most surprising involves microscopic algae known as diatoms. These single-celled organisms build intricate, glass-like shells made of biogenic silica, and while they’re alive, they play a major role in absorbing carbon dioxide, producing oxygen, and supporting marine food webs. But what happens after they die has turned out to be far more important—and much faster—than scientists ever believed.
A new study led by researchers at Georgia Tech has revealed that diatom shells can transform into authigenic clay minerals in as little as 40 days. This is a stunning revision of earlier assumptions. Not long ago, scientists thought this transformation—part of a process known as reverse weathering—took hundreds to thousands of years. Even the most recent estimates had brought the timescale down only to a few years. Now, thanks to detailed laboratory experiments, the timeline has collapsed to a few weeks.
This discovery not only changes our understanding of how the ocean processes silica, iron, aluminum, carbon, and trace metals, but also forces a rethinking of how the ocean influences long-term climate regulation.
Below is a clear breakdown of what the researchers found, why it matters, and what it means for our planet.
The Fast and Unexpected Transformation of Diatom Silica
When diatoms die, their beautiful silica skeletons sink to the seafloor. Traditionally, researchers believed that most of this silica simply dissolved and eventually returned to seawater, while the rest accumulated as buried sediment. But it turns out there’s another pathway.
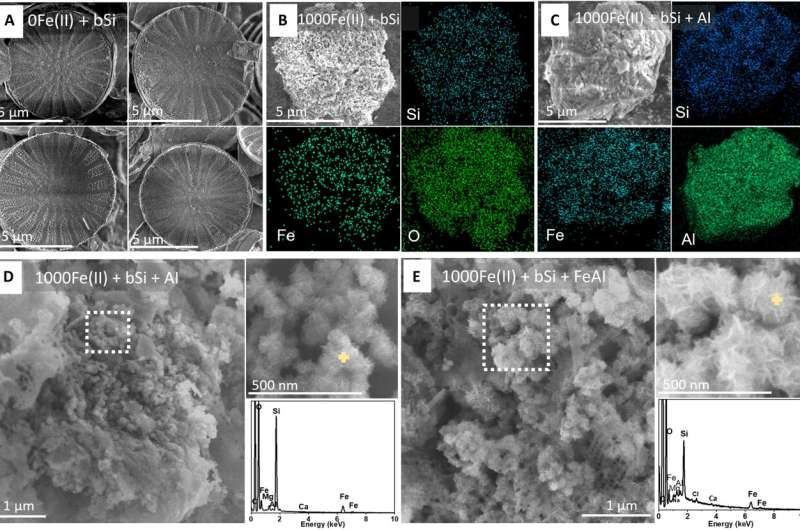
Some of the silica undergoes reverse weathering, a process where it reacts with metals like iron and aluminum in seawater to form new clay minerals. The major revelation from the Georgia Tech study is how rapidly this happens.
To test it, the team used a custom two-chamber reactor that mimicked seafloor conditions:
- One chamber contained diatom silica (bSi)
- The other held iron and aluminum minerals
- A thin membrane between them allowed dissolved elements to move back and forth
- Different concentrations of Fe(II) were tested, including 0 µM and 1000 µM
- Some experiments also included aluminum hydroxide and iron-aluminum combinations
Using advanced microscopy, spectroscopy, and chemical analysis, the researchers watched the entire transformation unfold—from the early dissolution of diatom silica to the appearance of brand-new, flake-shaped clay minerals.
After 40 days, the changes were unmistakable:
- With 0 µM Fe(II), nothing significant happened. The silica skeletons looked unchanged.
- With 1000 µM Fe(II), the diatom shells were extensively altered, showing clear associations of iron, silicon, and oxygen.
- When both iron and aluminum were present, the transformation was even more dramatic.
- The new minerals formed rosette-like clusters containing iron, silicon, aluminum, magnesium, and small amounts of calcium—matching the characteristics of naturally occurring marine clays.
These results align with what scientists have observed in marine sediments, but now we know how fast it can happen.
Why This Matters for the Ocean and the Climate
Reverse weathering doesn’t just reshape minerals—it reshapes the global carbon cycle.
Here’s how:
- Diatoms use silica to grow.
If silica is rapidly converted to clays, less of it remains available for new diatom growth. - Reverse weathering releases carbon dioxide.
During the formation of clay minerals, reactions between silica, bicarbonate, and dissolved metals can push CO₂ back into seawater. - That affects alkalinity and pH.
Reverse weathering reduces seawater alkalinity, which decreases its ability to absorb CO₂ from the atmosphere. - This means the process is tightly linked to climate.
Faster reverse weathering means the ocean can respond to environmental changes (like shifts in nutrient supply, river input, or sedimentation) on much shorter timescales.
The study therefore suggests that the seafloor isn’t just a passive place where minerals quietly accumulate. It’s a dynamic chemical engine, constantly influencing global cycles in ways we’re only now beginning to understand.
Solving a Long-Standing Silica Mystery
For years, oceanographers puzzled over a major imbalance: more silica enters the ocean than exits through burial. The numbers never quite added up.
This study offers a compelling explanation.
If diatom silica is rapidly turning into clay minerals instead of dissolving or being buried unchanged, then much of that silica simply “disappears” from the traditional accounting. Rapid reverse weathering could be the missing mechanism that keeps the ocean’s silica levels in balance.
What’s Happening at the Atomic Scale?
The research team found that the silica dissolution and transformation occur through molecular-scale interactions between dissolved silica, iron(II), aluminum, and other ions. Over time, these elements reorganize into stable clay minerals that resemble smectites or mica-like phases.
Key details include:
- The transformation needs both silica and metal cations.
- Higher Fe(II) concentrations drastically increase reaction speed.
- Aluminum helps stabilize the forming clays.
- The resulting minerals match those commonly found in marine sediments.
Even though these processes occur at microscopic scales, their planetary impact is enormous.
Why the Ocean Might Be More Sensitive to Change Than We Thought
If reverse weathering can shift dramatically based on iron levels, aluminum availability, or seawater composition, then:
- Changes in river runoff (which delivers silica, iron, and aluminum)
- Variations in coastal chemistry
- Changes in oxygen levels in sediments
- Shifts in diatom productivity due to warming
…could all alter how much carbon the ocean stores or releases.
This study suggests that modern environmental changes could affect these reactions much more quickly than previously assumed.
Additional Background: What Are Diatoms?
Diatoms are among the ocean’s most important organisms, responsible for:
- A large fraction of the ocean’s primary production
- Producing about 20% of Earth’s oxygen
- Driving the biological carbon pump, which transfers CO₂ to the deep ocean
They build their ornate shells—known as frustules—from silica, and these shells have fascinated scientists and artists for centuries because of their geometric beauty.
Diatoms thrive in nutrient-rich waters and form the base of countless food webs, feeding organisms from tiny zooplankton to huge whales.
The fact that even their leftover shells help regulate global chemistry shows how deeply interconnected ocean life and Earth’s climate system really are.
What Comes Next for This Line of Research
The scientists plan to expand their research by:
- Testing how changing seawater chemistry affects clay formation
- Studying sediments from both coastal and deep-sea environments
- Exploring how natural conditions compare to laboratory results
The ultimate goal is to help climate modelers better understand the ocean’s buffering systems, alkalinity cycles, and long-term CO₂ regulation.
As the lead researchers emphasized, even tiny reactions happening on the scale of microscopic particles can have effects that ripple all the way up to planetary climate patterns.
Research Paper
Rapid Transformation of Biogenic Silica to Authigenic Clay: Mechanisms and Geochemical Constraints
https://www.science.org/doi/10.1126/sciadv.adt3374