New Forecasting Tool Sharply Improves Epidemic Peak and Hospital Demand Predictions
Predicting when an epidemic will peak, how many people will need hospital care, and how long that critical period will last has always been one of the hardest challenges in public health. But a new forecasting method developed by researchers at the University of Texas at Austin could change that — offering a smarter, more reliable way to anticipate hospital demand during outbreaks.
Their new method, called “epimodulation,” enhances traditional forecasting models by teaching them to recognize the natural evolution of epidemics — especially the moment when cases stop rising and begin to fall. The work, led by Lauren Ancel Meyers and her team at UT Austin’s epiENGAGE center, was published in the journal Proceedings of the National Academy of Sciences (PNAS) on October 25, 2025.
The Core Idea: Teaching Models How Epidemics Behave
Most disease forecasting tools — especially data-driven or machine-learning ones — simply project recent trends forward. They look at how quickly cases are rising or falling and assume that pattern will continue. This works well for short-term changes, but fails near the peak, when cases start to slow down because of rising immunity, behavior change, or interventions.
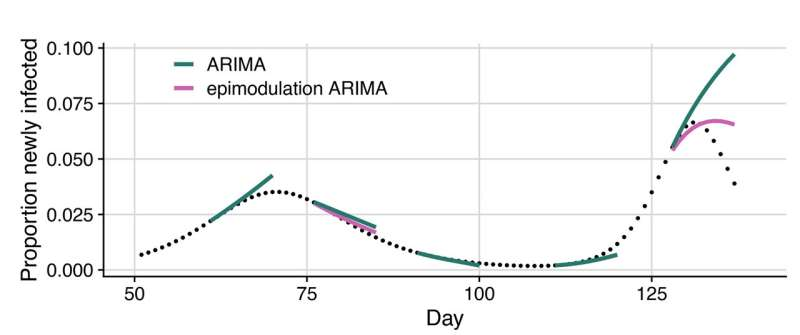
That’s where the new approach shines. Epimodulation adds a layer of epidemiological understanding to standard models, such as ARIMA (AutoRegressive Integrated Moving Average) models that are commonly used in outbreak forecasting. Instead of just fitting a curve to the data, the model now “expects” the epidemic curve to bend as people gain immunity or change behavior.
By doing so, the epimodulated model can detect early signs of slowdown and predict the turning point of an outbreak — something that most existing models struggle to capture.
How Epimodulation Works
The process is elegantly simple. Researchers start with a traditional model, such as ARIMA or Holt-Winters, and augment it with an additional parameter — represented by a value called θ (theta) — that captures the “bending” effect of an epidemic curve. This parameter allows the model to recognize when exponential growth begins to taper off.
For example:
- During the early phase of an epidemic, θ is near zero, meaning growth continues unhindered.
- As immunity builds and transmission slows, θ increases, causing the model to “bend” its forecast downward, anticipating a slowdown or decline.
The researchers tested this approach on both synthetic simulations (two-wave epidemics) and real-world datasets from past outbreaks, including COVID-19 and influenza hospital admissions.
Real-World Testing: COVID-19 and Influenza Forecasts
The team applied epimodulation to thousands of real forecasts:
- COVID-19 hospital admissions: Data from October 2020 to September 2023 across U.S. states.
- Influenza hospital admissions: Data from January 2022 to May 2023.
They tested how well epimodulated models performed for forecasts ranging from 1 to 28 days ahead, comparing them to standard versions of ARIMA, Holt-Winters, spline-based models, and even ensemble models used in the U.S. COVID-19 Forecast Hub.
The results were striking. Across nearly all models and diseases, forecast accuracy improved substantially, particularly at the epidemic peak — the moment when forecasting errors are usually the largest and most costly.
Quantifying the Improvements
Here’s what the researchers found:
- For COVID-19 hospital admissions:
Accuracy improved by 9–12% overall, and up to 25% during peak periods. - For influenza admissions:
Improvements ranged from 17–23% overall and up to 43% during peaks. - For ensemble models:
Even complex, combined forecasts showed meaningful gains — though smaller, since ensembles are already optimized from multiple models.
In one press release, the researchers noted that epimodulation led to up to 55% better accuracy during the peak of hospital demand forecasts. In other words, this technique doesn’t just polish forecasts slightly — it can dramatically sharpen them exactly when healthcare systems need reliable insight the most.
Why Epidemic Peaks Are So Hard to Predict
Understanding why forecasting peaks is difficult requires a quick look at how epidemics unfold. Outbreaks typically follow a recognizable pattern:
- Rapid growth phase: Infections rise exponentially as most people are still susceptible.
- Slowdown phase: Immunity builds and transmission opportunities decline.
- Peak and decline: The number of new infections levels off and begins to fall.
However, most machine learning and statistical models don’t “know” this biology. They just analyze data patterns — which means that if cases have been rising for weeks, they assume they’ll continue to rise. That leads to overestimation near the peak and underestimation afterward.
Epimodulation addresses this flaw by embedding basic epidemiological logic into the model — essentially teaching it to anticipate the slowdown before it becomes obvious in the data.
Beyond COVID-19: Broader Applications
The UT Austin team believes epimodulation can be used far beyond COVID-19 and flu. Many infectious diseases exhibit wave-like behavior, including Ebola, Mpox, and bird flu. Even new pathogens that have not yet emerged could benefit from this modeling approach.
That’s because the same underlying principle — population immunity and behavioral adaptation — governs most epidemics. Whether people become immune through infection or vaccination, or change behavior due to risk awareness, the epidemic curve bends for similar reasons.
This means that the model doesn’t need to know the biology of a specific virus. It only needs to recognize the general pattern of how transmission slows down over time.
What This Means for Healthcare and Policy
For hospitals, this innovation could be a game-changer. Predicting the peak of patient demand helps administrators decide when to:
- Scale up staffing and bed capacity.
- Prepare ICU resources and medical supplies.
- Manage elective procedures to free up space.
Better forecasts mean more efficient allocation of limited healthcare resources. They can also support public health agencies in communicating risk and preparing interventions before the system is overwhelmed.
For policymakers, it offers a more realistic real-time view of the epidemic’s trajectory. Instead of reacting to surprises, they can plan interventions with confidence — knowing roughly when and how big the peak will be.
Limitations and Future Directions
While epimodulation is promising, it’s not a magic solution. It performs best when the epidemic follows a clear wave pattern. In real life, outbreaks may overlap, or external factors such as new variants, weather, or behavior changes can create irregular shapes.
The technique also depends on high-quality data. Incomplete or delayed hospital reports can still throw off predictions. Moreover, most of the validation so far has been retrospective, meaning it was tested on past data where the outcome is already known. Real-time forecasting can be trickier due to delays and uncertainty.
That said, the researchers see this as a flexible framework that can plug into many existing models with minimal changes. In other words, you don’t have to build a new forecasting system from scratch — you can enhance the one you already have with epimodulation.
The Bigger Picture: Forecasting as a Tool for Resilience
The COVID-19 pandemic exposed just how crucial accurate forecasting is for healthcare systems. From ICU shortages to vaccine rollout timing, decisions depended on how well we could predict the future.
The emergence of epimodulation is part of a broader trend toward hybrid forecasting — blending machine learning’s pattern-recognition strengths with epidemiology’s biological realism. This combination is proving essential in preparing for the next pandemic, especially as climate change, urbanization, and global mobility increase outbreak risks.
By making forecasting models more “aware” of epidemic dynamics, epimodulation helps bridge the gap between data science and disease science — and that could make all the difference when the next major outbreak arrives.
Research reference:
Graham C. Gibson et al., Improving Outbreak Forecasts Through Model Augmentation, Proceedings of the National Academy of Sciences (2025).
https://doi.org/10.1073/pnas.2508575122