A High-Fat Diet Might Help Protect the Brain: University of Missouri Study Explores How Keto Could Slow Alzheimer’s Risk

Researchers at the University of Missouri (Mizzou) have uncovered intriguing evidence that a high-fat, low-carbohydrate diet—better known as the ketogenic diet—could play a role in protecting brain health and potentially lowering the risk of Alzheimer’s disease. Their new study, published in the Journal of Neurochemistry in September 2025, explores how this diet affects brain energy levels, gut bacteria, and genetic risk factors linked to cognitive decline.
The findings come from a collaboration inside Mizzou’s Roy Blunt NextGen Precision Health building, where Professor Ai-Ling Lin and doctoral student Kira Ivanich are leading research on the intersection of diet, brain metabolism, and genetics. Their focus is on how the APOE4 gene, the strongest known genetic risk factor for late-onset Alzheimer’s disease, interacts with diet and biology—especially in women.
Understanding the Premise: Why Brain Fuel Matters
The human brain primarily runs on glucose, a sugar derived from carbohydrates. However, in people who carry the APOE4 gene, especially women, the brain becomes less efficient at using glucose as fuel as they age. When this energy shortage occurs, neurons may start to malfunction, potentially paving the way for Alzheimer’s disease or other cognitive problems.
This is where the ketogenic diet comes in. By drastically reducing carbohydrate intake and increasing fats, the body shifts into a metabolic state called ketosis, producing ketones as an alternative fuel source. Ketones can cross the blood–brain barrier and serve as an efficient energy supply for brain cells. The Mizzou researchers wanted to see whether this metabolic switch could benefit those with the APOE4 gene.
The Study: Diet, Genes, and Gut Bacteria
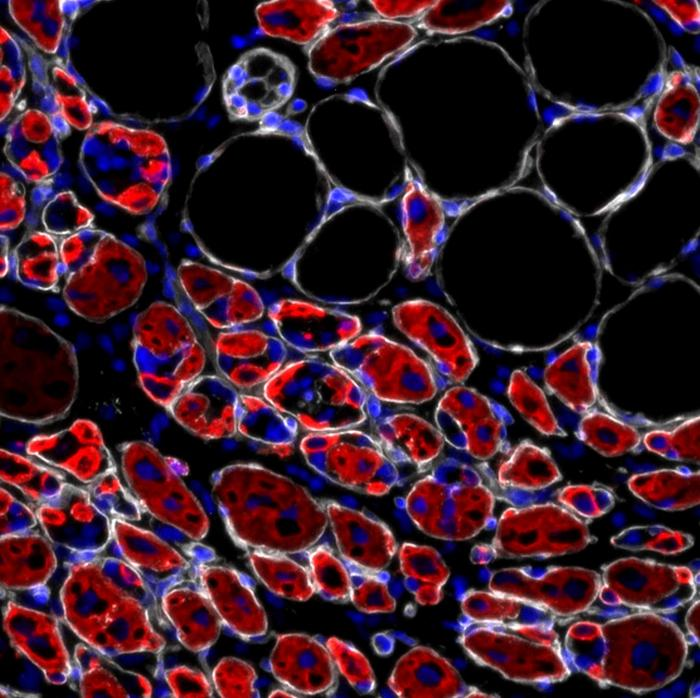
In their study, Lin and Ivanich used genetically engineered mice carrying the human APOE4 gene, as well as mice with the APOE3 gene, which poses little Alzheimer’s risk. Both male and female mice were included to see whether sex differences played a role in how the diet worked.
The experiment lasted 16 weeks, during which one group of mice was fed a standard control diet high in carbohydrates, while the other group followed a ketogenic diet rich in fats. The researchers then used advanced tools like metagenomic sequencing and targeted brain metabolomics to measure changes in both gut microbiota and brain chemistry.
What They Found: A Different Story for Males and Females
The results were clear and fascinating. Female APOE4 mice that ate the ketogenic diet developed healthier gut bacteria and showed higher levels of brain energy metabolites compared to those eating the standard diet. The shift in gut microbes was not random—it favored beneficial bacterial species such as Lactobacillus johnsonii and Lactobacillus reuteri, while reducing potentially harmful bacteria like Bacteroides intestinalis.
These gut changes were mirrored by improvements in the brain. Under the regular diet, APOE4 females showed deficiencies in several brain chemicals tied to mitochondrial function, neurotransmitter balance (such as glutamate and GABA), and redox metabolism, which is crucial for managing oxidative stress. On the ketogenic diet, many of these brain metabolites returned to healthier levels, suggesting the diet helped restore brain energy balance.
Interestingly, male APOE4 mice and mice with the APOE3 gene didn’t experience the same benefits. While their gut bacteria also changed under the ketogenic diet, their brain metabolism remained largely unchanged. This points to a sex- and genotype-specific effect—the diet seems to particularly help females with the APOE4 gene, who are already at higher risk for Alzheimer’s disease.
The Bigger Picture: Precision Nutrition and Personalized Brain Health
These findings emphasize the growing importance of precision nutrition, a field that focuses on tailoring diets to individual biology. Instead of assuming one diet fits all, the Mizzou team argues that factors like genes, gender, gut microbiome composition, and age should guide dietary recommendations.
According to the researchers, Alzheimer’s symptoms typically appear after age 65, meaning preventive strategies should begin long before cognitive decline sets in. Diets like the ketogenic plan might serve as an early intervention tool for people genetically predisposed to brain energy problems.
The researchers are quick to note that the goal isn’t to push everyone onto keto. Rather, it’s about identifying who stands to gain the most from such dietary strategies and how they might complement other lifestyle or medical interventions.
The Science Behind Ketones and Brain Energy
The science supporting ketone metabolism as a brain fuel source is well established. In ketosis, the liver breaks down fats into beta-hydroxybutyrate (BHB) and acetoacetate, which can be used by the brain when glucose is scarce. Unlike glucose, ketones produce less oxidative stress, offering cleaner energy and possibly reducing inflammation in neural tissue.
Previous studies have hinted that ketogenic diets may improve mitochondrial efficiency, enhance synaptic function, and reduce amyloid-beta accumulation, one of the hallmarks of Alzheimer’s disease. The Mizzou research builds on this by adding another layer: gut-brain communication.
The so-called gut–brain axis has become a major focus in neuroscience. Gut microbes produce metabolites and neurotransmitter-like compounds that can influence mood, cognition, and even neurodegeneration. By reshaping the gut microbiota, the ketogenic diet might be indirectly fine-tuning how the brain functions at a chemical level.
Why Gut Bacteria Matter
The gut microbiome plays a powerful role in regulating metabolism, immune responses, and brain chemistry. Imbalances in gut bacteria—known as dysbiosis—have been linked to neurological disorders, including depression, Parkinson’s, and Alzheimer’s disease.
In this study, the ketogenic diet helped restore a more diverse and stable microbiome in female APOE4 mice. Diversity is key because it reflects a healthy, resilient microbial ecosystem. Certain beneficial bacteria produce short-chain fatty acids (SCFAs), which can cross into the bloodstream and influence inflammation and metabolism in the brain.
These findings suggest that dietary interventions targeting the microbiome might one day become part of preventive brain health strategies, particularly for people with genetic risks.
Why the Differences Between Males and Females?
One of the most intriguing aspects of this study is that only females seemed to benefit from the ketogenic diet. The reasons aren’t fully understood, but scientists suspect several factors may contribute:
- Hormonal influence: Estrogen affects metabolism, inflammation, and the microbiome, potentially amplifying the benefits of ketones in females.
- Genetic interactions: The APOE4 gene may interact differently with metabolic pathways in male and female brains.
- Energy needs: Female brains sometimes rely more heavily on fat metabolism, making the switch to ketone fuel more natural or beneficial.
These differences highlight why both sex and genetics must be considered in nutritional neuroscience research—a one-size-fits-all model doesn’t work.
What This Means for Human Health
Before anyone rushes to overhaul their diet, it’s crucial to note that this was a mouse study, not a human clinical trial. While the results are promising, we don’t yet know whether the same effects occur in humans. Still, the groundwork laid here paves the way for future trials involving human APOE4 carriers, especially women.
The Mizzou team hopes their work will help define early prevention strategies for Alzheimer’s, long before symptoms appear. With advanced imaging technology and cross-disciplinary collaboration, they plan to move from preclinical mouse models to human trials in the coming years.
The Role of the NextGen Precision Health Building
This research was made possible thanks to the state-of-the-art imaging and molecular tools housed in Mizzou’s NextGen Precision Health building. The facility enables researchers to conduct every stage of their investigation—from molecular analysis to preclinical modeling—under one roof.
By keeping both research and clinical spaces closely linked, scientists can move discoveries more quickly from the lab bench to real-world applications. The project is a prime example of team science, combining expertise in neuroscience, metabolism, and genetics to address one of the world’s most challenging diseases.
The Human Connection
For doctoral student Kira Ivanich, this research is deeply personal. Her interest in Alzheimer’s prevention began when her grandmother developed the disease. That experience fueled her determination to explore how lifestyle changes—like diet—could protect brain health.
Working alongside Professor Ai-Ling Lin, she continues to explore how precision nutrition might give people a practical, science-backed way to preserve their cognitive abilities for as long as possible.
What You Can Take Away
The takeaway isn’t that everyone should immediately start a ketogenic diet. Instead, the study reminds us that brain health starts early, and diet plays a bigger role than most people realize. For individuals with a family history of Alzheimer’s—or known genetic risk factors like APOE4—monitoring diet, maintaining a healthy gut microbiome, and supporting metabolic health could make a real difference.
Future studies will likely determine the exact balance of fats, carbohydrates, and nutrients needed to achieve these benefits in humans. Until then, this research provides a hopeful glimpse into how personalized nutrition might one day become a cornerstone of Alzheimer’s prevention.