A Mother’s Circadian Rhythm May Predict Her Child’s Vulnerability to Bacterial Infection
New research is shedding light on a surprisingly powerful and often overlooked influence on immune health: a mother’s circadian rhythm, or internal biological clock. Scientists at The University of Texas MD Anderson Cancer Center have discovered that a mother’s circadian timing can shape the immune system of her offspring in ways that accurately predict how vulnerable that offspring will be to bacterial infections.
The study offers fresh insight into non-genetic factors that influence immune defenses and helps explain why individuals with very similar genetics and environments can experience vastly different infection outcomes. The findings were published in the journal Science Advances and were led by Alejandro Aballay, professor of Genetics and dean of the UTHealth Houston Graduate School of Biomedical Sciences.
At its core, the research highlights how biological timing—rather than DNA alone—can create meaningful differences in immune responses, potentially affecting how patients respond to infections during illness or medical treatments.
Understanding Circadian Rhythms and Immunity
Circadian rhythms are 24-hour internal cycles that regulate many physiological processes, including sleep, hormone release, metabolism, and immune function. These rhythms help organisms anticipate daily environmental changes and respond appropriately. Disruptions to circadian rhythms are already known to affect health, contributing to problems like metabolic disorders, sleep disturbances, and weakened immune responses.
What makes this study particularly interesting is its focus on maternal circadian rhythms and how they influence immune characteristics in offspring. Rather than examining genetic inheritance, the researchers explored how internal biological clocks passed from mother to offspring can shape immune readiness even before an infection occurs.
Why Identical Organisms Can Respond Differently to Infection
One of the longstanding mysteries in immunology is why individuals who are genetically similar—or even genetically identical—can show dramatically different responses to the same infection. This phenomenon is known as phenotypic heterogeneity, meaning observable differences in traits that cannot be explained solely by genetics or environment.
To investigate this, the researchers used Caenorhabditis elegans (C. elegans), a microscopic roundworm commonly used in laboratory research. These organisms are ideal for such studies because they can be bred to be nearly genetically identical and raised under tightly controlled environmental conditions.
Despite these similarities, the researchers observed striking differences in immune responses when the worms were exposed to bacterial infection. Some individuals were highly susceptible, while others were far more resilient.
Tracking Immune Responses With Fluorescent Biomarkers
To understand what caused these differences, the research team used fluorescent reporters to monitor immune biomarkers in the worms before and after bacterial exposure. These reporters allowed scientists to visualize immune activity in real time and measure baseline immune states prior to infection.
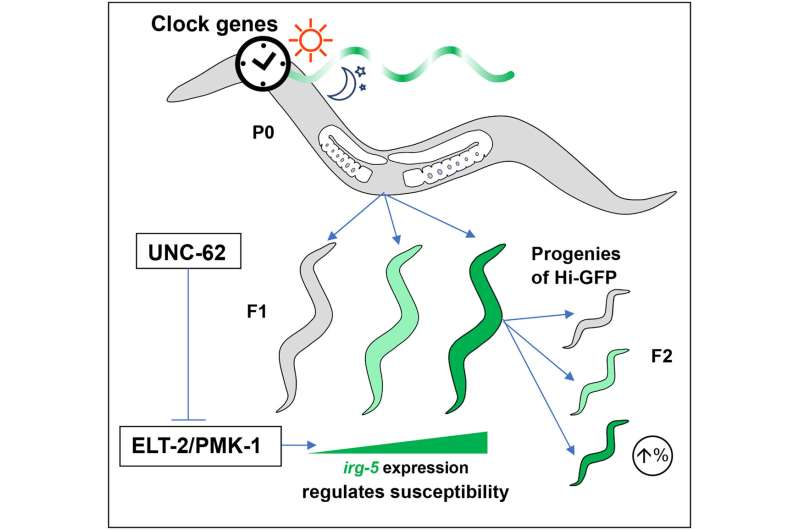
The results showed that worms with higher baseline expression of an inflammation-related biomarker were actually more susceptible to bacterial infection. This finding may seem counterintuitive at first, but it suggests that elevated baseline inflammation does not necessarily equate to stronger protection and may instead reflect an immune state that is less adaptable when infection strikes.
The researchers also identified key molecular targets within the signaling pathways that regulate this immune biomarker, helping clarify how immune variability arises at a mechanistic level.
The Role of the Mother’s Internal Clock
One of the most significant discoveries came when the researchers traced the source of immune variability back to maternal circadian rhythms. They found that the timing of a mother’s internal clock strongly influenced the baseline immune state of her offspring.
In other words, the mother’s circadian rhythm helped determine how active certain immune pathways were in the offspring even before exposure to bacteria. This baseline immune state turned out to be a reliable predictor of whether the offspring would successfully fight off infection or succumb to it.
To confirm this link, the researchers disrupted genes involved in the circadian clock. When these clock-related genes were interfered with, the maternal influence on immune variability disappeared. This result strongly suggests that circadian regulation is a major driver of immune diversity, independent of genetic differences.
Why This Matters for Human Health
Although the study was conducted in C. elegans, its implications extend far beyond worms. Many core biological pathways involved in circadian regulation and immune signaling are highly conserved across species, including humans.
These findings help explain why patients with similar diagnoses, genetic backgrounds, and treatment plans can experience very different infection outcomes. Circadian rhythms may act as a hidden variable that influences immune readiness and infection risk in ways that are not currently accounted for in clinical practice.
This research also raises important questions about how circadian disruptions—such as those caused by shift work, irregular sleep schedules, or chronic stress—might affect immune health across generations.
Circadian Rhythms as a Non-Genetic Inheritance Mechanism
One of the most intriguing aspects of the study is its demonstration that non-genetic factors can be transmitted from parent to offspring and have lasting biological effects. Unlike mutations or inherited genes, circadian influences operate through timing, regulation, and physiological state.
This opens the door to a broader understanding of inheritance, where biological rhythms, metabolic states, and environmental timing can shape health outcomes without altering DNA sequences.
Implications for Personalized Medicine
The findings suggest that circadian rhythms could eventually play a role in personalized medicine. If similar mechanisms are confirmed in humans, it may become possible to assess infection risk or immune resilience based on circadian profiles.
In the future, clinicians might consider not just genetic risk factors, but also biological timing, when predicting patient outcomes or designing treatment schedules. This could be especially relevant in cancer care, where infections are a major concern during immunosuppressive therapies.
A Growing Field: Chronoimmunology
This study contributes to a growing field known as chronoimmunology, which explores how circadian rhythms influence immune responses. Previous research has shown that the timing of vaccination, infection exposure, and even medication administration can affect immune effectiveness.
By identifying maternal circadian rhythms as a source of immune variability, this research adds another layer to our understanding of how time shapes biology.
What Comes Next
While more research is needed to determine how these findings translate to humans, the study provides a strong framework for exploring circadian regulation as a key factor in immune diversity. It also highlights the importance of considering biological timing when studying disease susceptibility and treatment response.
Ultimately, this work challenges the idea that genetics and environment alone determine health outcomes. Instead, it points to circadian rhythms as a powerful, predictive force shaping immune defenses across generations.
Research paper: https://www.science.org/doi/10.1126/sciadv.adx8112