A Newly Identified Enzyme Could Open the Door to Treating Degenerative Joint Diseases
Degenerative joint diseases such as osteoarthritis and intervertebral disc degeneration affect hundreds of millions of people worldwide. These conditions lead to chronic pain, stiffness, inflammation, and reduced mobility, and current medical treatments mostly revolve around symptom management rather than addressing the underlying biological causes. A new study from Yale University, published in Bone Research in 2025, presents a promising shift in this landscape by identifying the enzyme cytosolic phospholipase A2, or cPLA2, as a key driver of cartilage breakdown and joint degeneration. Researchers suggest that targeting this enzyme could be the foundation for developing meaningful, disease-modifying therapies.
Below is a clear breakdown of every specific detail revealed in the study, along with helpful background sections to deepen understanding of how joint degeneration works and why this enzyme matters.
Understanding cPLA2 and Why It Matters
The enzyme cPLA2 is already known for its involvement in inflammatory processes. It is responsible for producing arachidonic acid, a molecule that fuels the creation of many inflammatory mediators in the body. While its role in general inflammation has been understood for years, what has remained unclear until now is how directly it influences cartilage health — particularly the cells that maintain cartilage, known as chondrocytes.
The Yale researchers set out to explore this question in depth. Using a combination of single-cell RNA sequencing, human cartilage samples, genetic models, and drug-based inhibition, they determined that cPLA2 is not simply associated with inflammation but is also a major contributor to cartilage decay, chondrocyte aging, and structural degeneration of joints.
Their findings paint cPLA2 as a central regulator of joint deterioration — a discovery with major implications for future therapies.
What the Researchers Found in Human Cartilage
Using advanced sequencing of human osteoarthritis and healthy cartilage tissues, the team identified eight distinct types of chondrocytes. Among these, two types stood out due to their strong association with degeneration:
- Prehypertrophic chondrocytes
- Fibrochondrocytes
Both of these cell subgroups showed significantly elevated levels of cPLA2. These cells were already predisposed to cartilage breakdown and displayed clear markers of aging and degeneration. The consistent pattern across multiple human samples indicates that cPLA2 is intimately tied to cartilage damage.
The lab also confirmed these findings in preclinical research, where the same elevated cPLA2 expression appeared in cartilage samples from mouse models of joint degeneration.
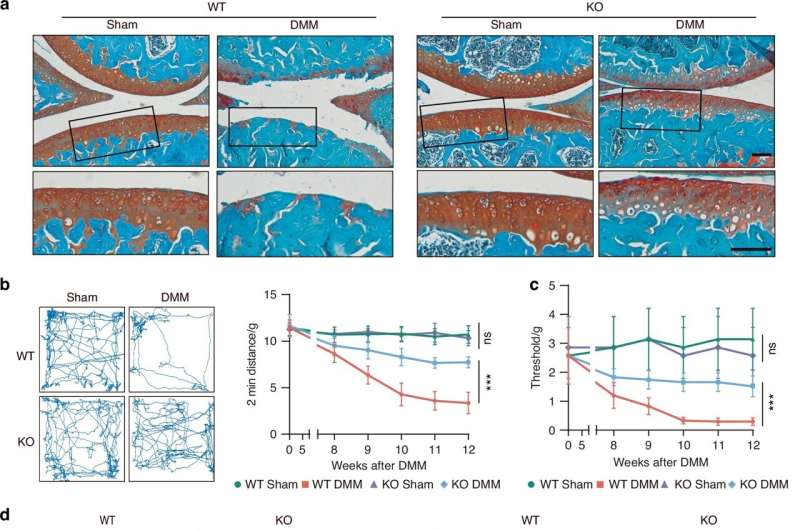
Genetic Deletion of cPLA2 Led to Dramatic Improvements
One of the most compelling parts of the study involved genetically altering mice to remove the cPLA2 gene. The results were striking:
- Major reductions in cartilage loss
- Lower levels of joint inflammation
- Less thickening of the subchondral bone layer beneath cartilage
- Reduced inflammation in the synovial membrane, the thin tissue that produces joint-lubricating fluid
- Fewer osteophytes, the bony growths commonly seen in osteoarthritis
- Improved pain levels and joint function
These changes suggest that cPLA2 isn’t just involved in degeneration — it actively fuels it. Removing or blocking the enzyme helps preserve joint structure and noticeably improves functional outcomes.
A Surprising Drug Candidate: Fexofenadine
An interesting twist in the study is the exploration of fexofenadine, a very common over-the-counter antihistamine widely used for allergies. Members of the Yale research team had previously identified the drug as a potential inhibitor of cPLA2.
In this study, fexofenadine performed impressively:
- It blocked cPLA2 activity in chondrocytes.
- It reduced inflammation in cartilage tissue.
- It prevented age-related changes in cartilage cells.
- It reduced cartilage degradation in multiple preclinical models.
- Its protective effects occurred only when cPLA2 was present, confirming that the drug’s benefits were directly tied to inhibiting this enzyme.
Because fexofenadine already has a well-established safety profile in humans, it becomes a promising starting point for rapid drug repurposing. It may not be the final form of a cPLA2-targeting therapy, but its involvement accelerates the path to clinical application.
Why Degenerative Joint Diseases Are So Difficult to Treat
Osteoarthritis and intervertebral disc degeneration are more complex than many people realize. They aren’t caused by a single factor but rather a combination of:
- Mechanical wear
- Genetic predispositions
- Inflammation
- Chondrocyte aging and senescence
- Metabolic and systemic factors
Most treatments — like pain relievers, injections, and physical therapy — focus on reducing discomfort while the disease continues progressing silently in the background. There has long been a search for disease-modifying treatments, which intervene in the actual biology of joint degeneration rather than simply masking symptoms.
The discovery that blocking cPLA2 helps maintain cartilage integrity, reduces inflammation, slows chondrocyte aging, and improves functional outcomes suggests that researchers may finally be on the right track.
Broader Implications Beyond Joint Diseases
One aspect the study emphasizes is the possibility that cPLA2 may contribute to other inflammatory or degenerative conditions throughout the body. Since the enzyme is deeply involved in inflammation and cellular aging, it could be a candidate for therapies targeting:
- Autoimmune diseases
- Other musculoskeletal disorders
- Certain degenerative conditions involving connective tissues
The Yale researchers specifically encourage expanded investigation into these possibilities, as understanding the broader molecular network of cPLA2 may unlock new therapeutic applications.
Additional Insight: How cPLA2 Affects Chondrocyte Aging
Chondrocyte aging, or senescence, is now understood to be a major driver of cartilage breakdown. Senescent cells don’t just stop dividing — they begin releasing pro-inflammatory molecules and enzymes that degrade cartilage matrix. This contributes to a cycle of deterioration.
According to the study:
- cPLA2 increases markers of chondrocyte senescence, such as p16 and p21
- Blocking cPLA2 reduces these markers
- This makes chondrocytes more stable, healthier, and more capable of maintaining cartilage tissue
The ability to reduce senescence is significant. Very few current treatments target cellular aging directly, and those that do typically remain experimental. This research brings senescence-related therapy one step closer to reality.
What Needs to Happen Next
Although the results are promising, this research is still at the preclinical stage. Before any therapy can reach patients, researchers need to conduct:
- Human safety studies specific to long-term cPLA2 inhibition
- Clinical trials assessing whether fexofenadine or other inhibitors reliably prevent joint degeneration
- Studies on how blocking cPLA2 affects other joint cell types
- Long-term evaluations of functional outcomes
Nevertheless, the data so far strongly supports continued development. For many people suffering from painful, progressive joint diseases, this work points toward a future in which treatments may not just mask symptoms but actually change the course of the disease.
Research Reference
Cytosolic phospholipase A2 as a therapeutic target for degenerative joint diseases
https://www.nature.com/articles/s41413-025-00470-9