A Promising New Antibody Approach Shows Potential to Stop Polycystic Kidney Disease at Its Source
Polycystic kidney disease (PKD) is one of those conditions that quietly progresses for years before causing life-altering complications. It’s a hereditary disorder where fluid-filled cysts form throughout the kidneys, gradually multiplying, expanding, and crowding out healthy tissue. Over time, these cysts can grow so large and so numerous that they impair kidney function, pushing many patients toward dialysis or even transplantation. And despite decades of research, there is currently no cure.
But a new study from researchers at UC Santa Barbara (UCSB) offers something genuinely exciting: a prototype therapy that finally reaches the inside of these cysts — the very place where disease-driving signals originate. The approach uses a specially engineered dimeric immunoglobulin A (dIgA) antibody to block cyst-growth pathways from within, something existing treatments have never been able to do.
Below is a clear, detailed breakdown of how this potential therapy works, what the researchers discovered, and what challenges still lie ahead.
Understanding Why PKD Is So Hard to Treat
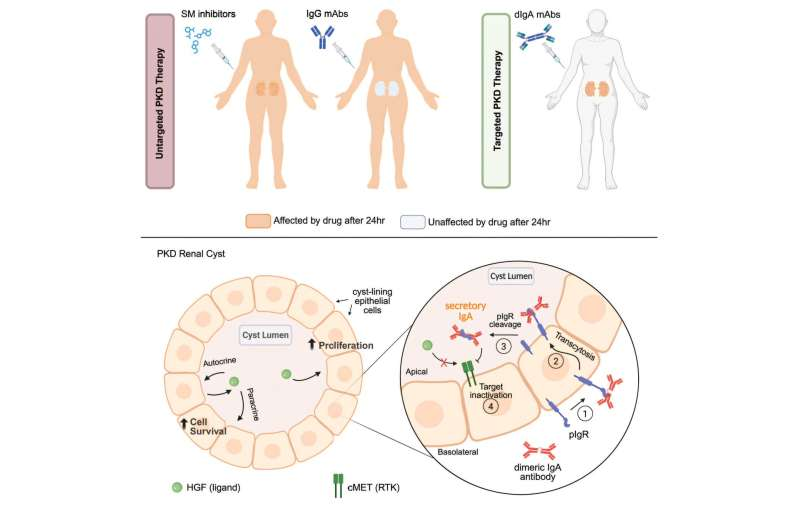
One of the biggest hurdles in PKD therapy is accessibility. The cysts are enclosed pockets lined by epithelial cells, and the signals that cause cysts to enlarge — especially growth factors secreted by cyst-lining cells — operate within the sealed cyst space. This means that drugs circulating in the bloodstream often can’t get inside where the real problem is happening.
Small-molecule drugs can sometimes penetrate better, but the only commercially available drug that slows PKD progression comes with significant side effects and toxicity risks for surrounding kidney tissues.
On the other hand, monoclonal antibodies offer highly targeted therapeutic action, but the most widely used type — immunoglobulin G (IgG) — is simply too large to cross epithelial barriers. It’s effective in cancer therapy, autoimmune diseases, and other conditions, but not here. IgG antibodies “stay outside,” while PKD pathology continues “inside” the cysts.
That barrier problem has been one of the field’s biggest frustrations.
The Idea: Use dIgA Antibodies That Naturally Cross Epithelial Layers
This is where UCSB researchers got creative. In nature, dIgA antibodies are produced in mucosal tissues, such as tears, saliva, and mucus. Their entire purpose is to cross epithelial surfaces as a first line of immune defense.
This happens through a receptor called polymeric immunoglobulin receptor (pIgR) — something epithelial cells express, including those lining PKD cysts.
Back in 2015, the Weimbs Lab suggested that this natural transport system could be repurposed. If researchers could turn an IgG antibody into a dIgA version, it could theoretically latch onto pIgR and be transported straight into cyst lumens. Once there, it could bind to whatever target drives growth inside the cyst environment.
In their new 2025 study, they put this idea to the test at full scale.
Engineering an Antibody That Targets cMET — A Driver of Cyst Growth
The team focused on cMET, a receptor involved in cell proliferation and commonly found active in PKD cysts. They:
- Altered the DNA sequence of a standard IgG antibody.
- Reworked its structure to give it a dIgA backbone, enabling epithelial transport.
- Confirmed that the engineered antibody still recognized and bound cMET correctly.
- Tested the antibody in cell cultures and then in mouse models of PKD.
This step was crucial because engineering antibodies is tricky — changing their structure can easily ruin their ability to bind their targets. But in this case, the new dIgA version remained fully functional.
What Happened in the Mice: Transport, Targeting, and a Surprising Additional Effect
The results were surprisingly strong.
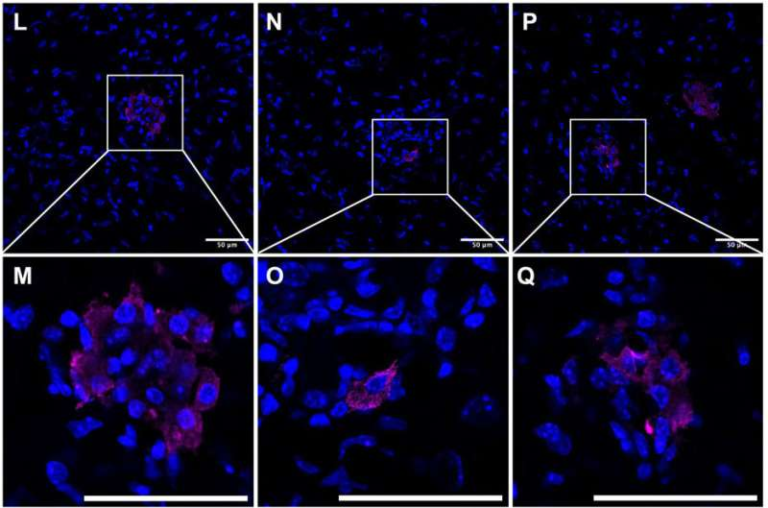
1. The dIgA antibody successfully entered the cysts.
As hoped, the antibody used the pIgR transport mechanism to cross the epithelial lining and accumulate directly in the cyst fluid.
2. Once inside, it inhibited the activity of cMET.
This is important because cyst-lining cells stimulate each other endlessly by secreting growth factors into the cyst fluid. Blocking cMET interrupts this autocrine/paracrine feedback loop.
3. Treated kidneys showed reduced cyst-growth signals.
Measurements showed a drop in cell proliferation inside cysts.
4. The therapy triggered a dramatic wave of apoptosis — but only in cyst cells.
Unexpectedly, the treatment caused a sharp increase in programmed cell death in the cyst epithelial cells while leaving healthy kidney tissue unharmed.
This kind of selective effect is rare and extremely valuable in therapy development.
5. No apparent toxic side effects were observed.
Throughout the study, healthy tissues appeared unaffected by the antibody.
The research is still in the preclinical stage, so humans won’t be receiving this therapy anytime soon. But the results demonstrate something important: it is possible to deliver a therapeutic antibody directly into PKD cysts and meaningfully impact disease progression.
What Makes This a Potential “Magic Bullet”?
Researchers sometimes hesitate to use terms like “magic bullet,” but in context, the concept makes sense:
- The antibody travels only where it needs to go.
- It targets a specific receptor driving cyst growth.
- It avoids healthy tissues, minimizing risk.
- It uses a natural transport system instead of brute-force penetration.
- It may eventually work in combination with other antibodies to target multiple pathways at once.
Because many different growth factors are active in PKD cysts, the researchers envision future therapies that mix several dIgA antibodies. This could help pinpoint which pathway affects cyst progression the most — or even reverse the disease in some cases.
Broader Implications for Antibody Therapy
What’s especially interesting is how adaptable this approach might be. Many existing monoclonal antibodies used for other diseases could theoretically be re-engineered into dIgA forms to cross epithelial barriers in other organs. This could open the door to treating diseases involving:
- lung mucosal surfaces
- gastrointestinal barriers
- epithelial-sealed cystic diseases
- chronic infections in epithelial compartments
If this technology proves scalable, it might become a platform, not just a single therapy.
Challenges the Researchers Still Face
Even with all the promising results, several hurdles remain:
- Finding pharmaceutical or biotech partners with the capacity to produce engineered dIgA antibodies at large scale.
- Conducting long-term toxicity studies.
- Identifying which receptors or growth factors should be targeted first in human trials.
- Comparing multiple antibodies head-to-head to determine the most effective combinations.
- Overcoming regulatory and manufacturing challenges — dIgA antibodies are inherently more complex than standard IgG therapies.
But the path is clearer than ever before, and the groundwork laid by this study makes it much more likely that PKD-focused antibody therapies will eventually reach clinical trials.
More About PKD for Curious Readers
PKD affects millions worldwide and comes in two major forms:
- Autosomal dominant PKD (ADPKD) – the most common form, symptoms appear in adulthood.
- Autosomal recessive PKD (ARPKD) – rarer and often more severe, appearing in childhood.
Cysts don’t just form in the kidneys — patients may develop them in the liver, pancreas, and other organs. Many also experience high blood pressure, abdominal pain, and recurrent infections.
The disease is driven by genetic mutations (often in PKD1 or PKD2) that affect how kidney cells grow and divide. Because the underlying signaling pathways are so complicated, treatments have been difficult to design. That’s why a method capable of directly entering cysts and shutting down self-stimulating growth loops is such a promising development.
Research Reference:
Development of a cyst-targeted therapy for polycystic kidney disease using an antagonistic dimeric IgA monoclonal antibody against cMET
https://doi.org/10.1016/j.xcrm.2025.102335