A Rare Genetic Mutation Shows Unusual Protection Against Alzheimer’s Disease in New Research

A new study from Rutgers University highlights a rare genetic mutation that appears to help the brain’s immune cells resist the harmful changes typically seen in Alzheimer’s disease, offering scientists a fresh direction for therapeutic development. Instead of focusing solely on clearing toxic proteins from the brain, researchers now see real potential in strategies aimed at strengthening the brain’s own defensive system. This discovery centers on a mutation in a gene called CSF2RB, specifically a variant known as A455D, and its remarkable behavior in microglia—the brain’s resident immune cells.
Understanding Why This Mutation Matters
Alzheimer’s disease is widely associated with toxic protein accumulation, chronic inflammation, and the slow breakdown of neuronal health. Microglia play a major role in this process because they act as the brain’s cleanup crew. When they function properly, microglia remove waste, clear harmful proteins, and maintain a balanced environment. But in Alzheimer’s, these cells often become overactivated and inflamed, leading to long-term damage instead of protection.
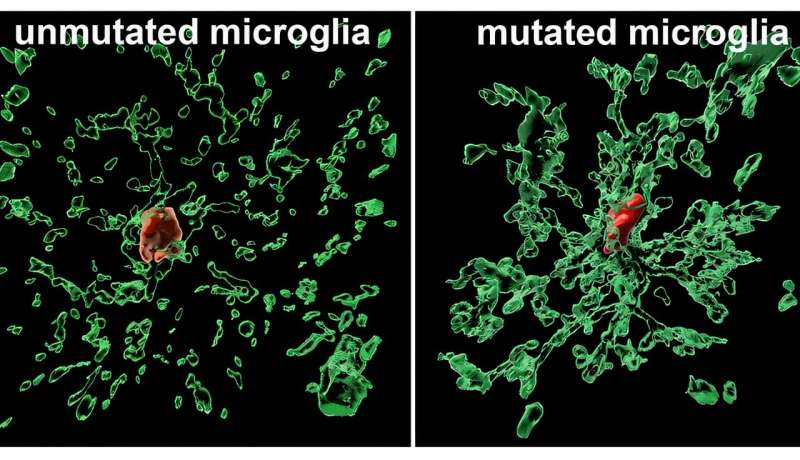
Credit: Jiang Lab/Rutgers University.
The Rutgers team, led by neuroscientist Peng Jiang and researcher Mengmeng Jin, turned their attention to individuals with Down syndrome, a population that almost always develops early-onset Alzheimer’s due to having three copies of chromosome 21. This chromosome includes key genes involved in toxic protein buildup. Despite this overwhelming risk, a small subset of people with Down syndrome never develop dementia even when their brains show Alzheimer’s-like pathology. This puzzling resilience prompted scientists to investigate possible genetic factors behind it.
One of those factors turned out to be the CSF2RB A455D mutation, found in immune cells of a few individuals in this unusually protected group. The researchers suspected this mutation might give microglia enhanced resilience, and the new study provides detailed evidence supporting that idea.
How Researchers Tested the Mutation
To understand what the CSF2RB A455D variant does, the team created human microglia using stem cell technology—some with the mutation and some without it. They transplanted these engineered human microglia into the brains of mice, creating a chimeric mouse model. This gave the team a way to observe human immune-cell behavior inside a living brain environment, a crucial step because microglia interact heavily with surrounding neurons and proteins.
Once the transplanted cells were in place, the mice were exposed to Alzheimer’s-related toxic proteins, allowing researchers to watch how the mutated microglia responded compared to normal ones.
The results were striking. Microglia carrying the A455D mutation stayed younger, healthier, and more resistant to chronic inflammation. Instead of becoming overactivated and damaging nearby neurons, they continued functioning effectively. These cells were better at clearing harmful proteins, including Alzheimer’s-linked proteins that typically overwhelm microglia over time.
In mixed environments—where both mutated and non-mutated microglia existed—another surprising pattern emerged: the mutated microglia gradually outcompeted and replaced the normal ones. While unmutated microglia weakened, the mutated cells remained stable and strong, essentially refreshing the local immune-cell population. This dominance effect appeared even when the mutation was introduced into cells from people outside the Down syndrome population, suggesting its protective benefit is not limited to one group.
Why the Mutation Helps: What the Study Suggests
Microglial aging and chronic inflammation play central roles in Alzheimer’s disease. The study found that the A455D mutation helps microglia avoid this damaging inflammatory cycle. The mutated cells did not undergo the typical long-term inflammatory activation that leads to cellular exhaustion. Instead, they kept functioning in a balanced state while continuing to clean up toxic protein deposits.
Because Alzheimer’s involves both protein aggregation and immune dysfunction, a cell type that can stay efficient under pressure represents a major therapeutic interest. It shifts attention from simply clearing proteins to building resilient brain environments, an approach that could complement existing therapies.
Potential New Treatment Strategies
The findings open the door to two promising therapeutic possibilities:
- Transplanting engineered microglia into patients
Scientists could potentially generate microglia carrying the A455D mutation and introduce them into a patient’s brain. Over time, these cells might replace vulnerable microglia and restore a healthier immune environment capable of combating Alzheimer’s pathology more effectively. - Using gene therapy to introduce the mutation directly
Instead of transplanting cells, gene-editing strategies could modify a person’s existing microglia to carry the protective mutation. This approach, if proven safe, could offer a more direct and scalable way to harness the mutation’s benefits.
Both strategies, however, require extensive testing. Any type of genetic modification or microglial replacement must be evaluated for long-term safety, stability, and potential side effects.
Broader Context: Protective Mutations in Alzheimer’s Research
The concept of protective genetic mutations is gaining more attention in neuroscience. While many studies focus on mutations that increase disease risk, there is growing interest in identifying mutations that do the opposite. These resilience-conferring variants may unlock entirely new therapeutic methods.
A famous previous example is a protective variant of the APOE gene, which reduces risk even in individuals who carry the high-risk APOE4 allele. The new CSF2RB A455D discovery fits this broader pattern and adds important insight into how the immune system influences Alzheimer’s progression.
What Makes This Discovery Stand Out
This mutation offers several unique insights:
- It highlights the crucial role of microglia, not just neurons, in Alzheimer’s disease.
- It demonstrates that immune resilience is possible even in environments heavily loaded with toxic proteins.
- It shows that a single mutation can dramatically influence cellular aging, inflammation, and competitive survival.
- It suggests that Alzheimer’s treatments could move beyond protein clearance and toward immune-system engineering.
For individuals with Down syndrome, understanding why some escape dementia despite extreme risk may lead to targeted interventions that benefit millions of others. For Alzheimer’s researchers, this work expands the list of biological mechanisms worth exploring—not just to slow the disease, but potentially to prevent it.
Additional Insight: Why Microglia Are Central to Brain Health
Microglia are increasingly recognized as key players in neurodegenerative diseases:
- They regulate synaptic pruning, ensuring neural networks remain balanced.
- They respond to injury and infection, making them essential guardians of the brain.
- When dysfunctional, they can promote inflammation and neuronal damage.
Because Alzheimer’s involves both toxic protein buildup and immune dysregulation, microglia sit at the center of both processes. A mutation that improves their resilience could influence several disease mechanisms at once, which is why this particular discovery is receiving strong attention.
Link to the Research Paper
A Myeloid Trisomy 21-Associated Gene Variant Is Protective From Alzheimer’s Disease
https://doi.org/10.1038/s41593-025-02117-8