AI Helps Scientists Identify a Key Mpox Protein That Could Transform Vaccines and Antibody Treatments
Artificial intelligence has once again shown how powerful it can be in modern biomedical research. This time, it has helped scientists identify a previously overlooked protein on the monkeypox virus (MPXV) that could become the foundation for new vaccines and antibody-based therapies against mpox. The findings come from an international research team led by scientists at The University of Texas at Austin and collaborators in Italy, and the study has been published in the journal Science Translational Medicine.
This discovery is particularly important because mpox remains a global public health concern, and existing vaccines—largely adapted from smallpox—are far from ideal in terms of cost, complexity, and accessibility.
How AI Changed the Game in Mpox Research
The monkeypox virus has a complex structure, with dozens of proteins on its outer surface. Some of these proteins are critical for infecting human cells, while others play supporting roles. Identifying which surface proteins are actually targeted by protective antibodies has traditionally been a slow and difficult process.
In this study, researchers turned to AlphaFold 3, an advanced AI model designed to predict protein structures and interactions. Instead of testing viral proteins one by one in the lab—a process that could take years—the AI rapidly analyzed interactions between known antibodies and viral surface proteins.
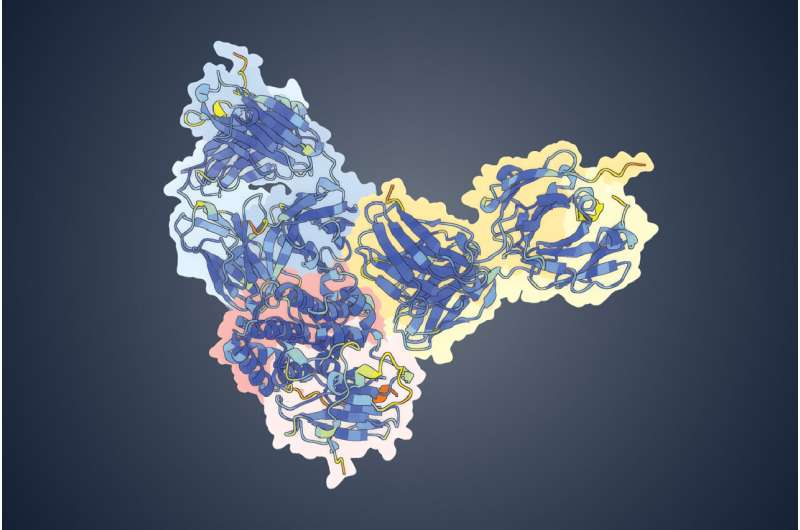
The result was a clear and confident prediction: several powerful neutralizing antibodies bind strongly to a viral surface protein known as OPG153.
What makes this especially notable is that OPG153 had never before been identified as a target for neutralizing antibodies. In other words, scientists were not even looking at this protein as a serious vaccine or therapy candidate until AI pointed them in that direction.
Where the Antibodies Came From
Before AI entered the picture, researchers had already made an important discovery. Scientists at the Fondazione Biotecnopolo di Siena in Italy analyzed blood samples from people who had either recovered from mpox or had been vaccinated against it. From these samples, they isolated 12 antibodies that were highly effective at neutralizing the virus.
However, there was a major missing piece of the puzzle: the team did not know which viral proteins these antibodies were targeting. With roughly 35 different surface proteins on MPXV, narrowing down the correct match was a significant challenge.
This is where the UT Austin team stepped in. By feeding antibody and viral protein data into AlphaFold 3, the researchers were able to quickly identify OPG153 as the most likely target—and laboratory experiments later confirmed the AI’s prediction.
Testing the Discovery in Mice
Identifying a promising target is only the first step. To see whether OPG153 could actually work as a vaccine component, the researchers engineered the protein and injected it into mice.
The results were encouraging. The mice produced strong neutralizing antibodies capable of blocking MPXV infection. This provided clear evidence that OPG153 is not just an interesting molecular target, but a functional antigen that can train the immune system to fight the virus.
This finding opens the door to both protein-based vaccines and antibody therapies that directly target OPG153.
Why This Matters for Vaccine Development
Current vaccines used during mpox outbreaks are based on live, weakened viruses originally designed to combat smallpox. While these vaccines can be effective, they are also:
- Complex to manufacture
- Expensive to produce
- Dependent on high-level biosafety facilities
- Less suitable for rapid, large-scale global distribution
By contrast, a vaccine based on a single viral protein like OPG153 could be simpler, cheaper, and faster to produce. Protein-based vaccines are already widely used for other diseases and are generally easier to scale up, especially in low- and middle-income countries.
This makes the OPG153 discovery particularly valuable from a global health perspective.
Reverse Vaccinology Explained
The approach used in this research is known as reverse vaccinology, a strategy that flips traditional vaccine development on its head.
Instead of starting with the virus and guessing which components might work as a vaccine, researchers begin with successful immune responses in humans. In this case, they studied antibodies naturally produced by people who had already fought off mpox or responded well to vaccination.
From there, scientists worked backward to identify the exact viral protein responsible for triggering those protective antibodies. Once identified, that protein was engineered and tested as a vaccine antigen.
This method is faster, more precise, and increasingly powerful when combined with AI tools like AlphaFold.
Implications Beyond Mpox
Monkeypox virus is closely related to the virus that causes smallpox, a disease that was eradicated decades ago but still poses a serious concern due to its potential use as a bioterrorism agent.
Because of this close relationship, insights gained from OPG153 could potentially help scientists develop next-generation vaccines or antibody treatments for smallpox as well. Any advancement that simplifies vaccine design for poxviruses has implications that go well beyond mpox alone.
Ongoing Research and Patents
The discovery of OPG153 has already moved beyond the lab bench. The University of Texas at Austin has filed a patent application covering the use of OPG153 and its modified forms as a vaccine antigen. Meanwhile, the Italian research team has filed a separate patent application related to antibodies that specifically target this protein.
The researchers are now focused on:
- Optimizing OPG153-based vaccine designs
- Improving the effectiveness of OPG153-targeting antibodies
- Reducing manufacturing costs
- Preparing candidates for future human trials
While clinical use is still some distance away, the groundwork has clearly been laid.
A Quick Look Back at the Mpox Outbreak
Mpox gained global attention in 2022, when the virus spread far beyond its historically endemic regions. The outbreak caused flu-like symptoms, painful skin lesions, and rashes, affecting more than 150,000 people worldwide and resulting in nearly 500 deaths.
Although emergency vaccination campaigns helped slow the spread, the outbreak highlighted major gaps in preparedness, particularly the lack of modern, targeted vaccines designed specifically for mpox.
The identification of OPG153 directly addresses this gap.
Why AI Is Becoming Essential in Virology
This study is another strong example of how artificial intelligence is accelerating biomedical discovery. Tasks that once required years of trial-and-error experimentation can now be completed in weeks or months with AI-guided predictions.
In viruses with complex structures like MPXV, AI tools help researchers focus their resources on the most promising targets, reducing costs and speeding up innovation. As AI models continue to improve, discoveries like this are likely to become more common.
Research Paper Reference
Antigen-agnostic identification of poxvirus broadly neutralizing antibodies targeting OPG153, Science Translational Medicine (2025)
https://www.science.org/doi/10.1126/scitranslmed.aeb3840