Biodegradable Microneedle Patch Shows Powerful Potential for Healing the Heart After a Heart Attack
A new development from researchers at Texas A&M University has introduced a promising way to support heart repair after a heart attack. The innovation is a biodegradable microneedle patch designed to deliver the healing molecule interleukin-4 (IL-4) directly into damaged heart tissue. What makes this approach stand out is how precisely it targets the injured muscle while avoiding effects on the rest of the body. Instead of using systemic injections—which can cause complications by affecting other organs—this patch provides highly localized treatment exactly where it’s needed.
At the center of this work is Dr. Ke Huang, an assistant professor in the university’s Department of Pharmaceutical Sciences. His team published the findings in Cell Biomaterials, presenting detailed results from both rodent and porcine (pig) models of myocardial infarction. The study highlights how the patch helps regulate immune activity, reduce inflammation, improve healing conditions, and ultimately support better heart function after injury.
How a Heart Attack Damages the Heart
A heart attack occurs when blood flow to a section of the heart becomes blocked. Without oxygen and nutrients, heart muscle cells begin to die. The body quickly responds by forming scar tissue, which stabilizes the damaged area but does not contract like healthy muscle. Over time, the remaining muscle must work harder, often leading to long-term decline in cardiac function and, eventually, heart failure.
This pattern of damage and compensation has historically been difficult to interrupt. Traditional treatments manage symptoms but cannot regenerate lost heart muscle. The new patch offers a way to intervene at the biological level by providing an environment that encourages repair instead of scarring.
What Makes This Patch Different
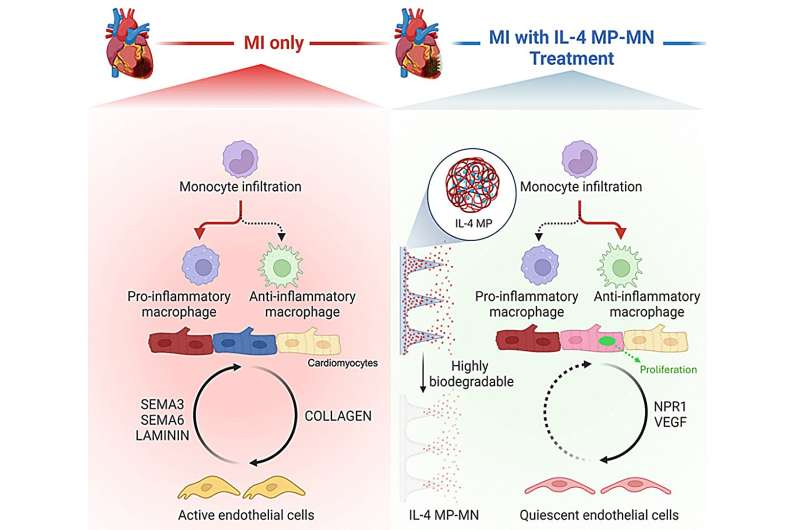
The patch uses a microneedle system, where each tiny needle contains PLGA microparticles loaded with IL-4. When applied to the surface of the heart, the biodegradable needles dissolve and gradually release IL-4 directly into the injured region. This direct access matters because penetrating the outer layer of the heart (the epicardium) and reaching the underlying muscle is typically very challenging.
IL-4 is known for its role in controlling immune reactions. In a healing context, it shifts immune cells—specifically macrophages—from a pro-inflammatory state to a pro-repair state. This shift reduces harmful inflammation and promotes tissue recovery. Delivering IL-4 locally is important because systemic IL-4 can produce unwanted effects throughout the body. The patch neatly avoids those risks by isolating the therapy at the injury site.
Another noteworthy detail is the hyaluronic acid (HA) hydrogel base that supports the microneedles. HA is biocompatible, helps maintain structural stability during application, and safely breaks down once its job is done. This means no follow-up surgery is needed to remove the patch.
Effects Observed in Animal Models
In both rodent and pig studies, the patch demonstrated several encouraging results:
- Reduced scar tissue formation
- Improved communication among heart cells, especially between cardiomyocytes and endothelial cells
- Decreased inflammatory signaling from endothelial cells
- Activation of the NPR1 signaling pathway, which supports blood vessel and cardiac health
- Better preservation of heart structure
- Improved functional recovery after myocardial infarction
One of the more intriguing observations was how the treated cardiomyocytes behaved. They didn’t just survive; they interacted more effectively with surrounding cells. Enhanced cellular communication is a key factor in long-term healing and may indicate improved regenerative potential.
The delivery of IL-4 through microneedles also created a more supportive microenvironment by promoting immune balance, reducing harmful inflammatory responses, and encouraging healthier tissue remodeling.
Why Targeted Immunomodulation Matters
Repairing the heart after a heart attack involves more than simply stopping cell death. The immune system plays a major role, and macrophages are crucial. These cells can either exacerbate inflammation or help resolve it. IL-4 pushes them toward a healing mode.
Past attempts to use IL-4 for cardiac repair failed largely because of systemic toxicity—meaning the molecule affected tissues far beyond the heart. The patch solves this problem by keeping IL-4 in the damaged region. This approach highlights a developing trend in regenerative medicine: localized therapeutic delivery, which allows for more potent effects with fewer risks.
Limitations and Future Improvements
The biggest current limitation is that the patch must be applied during open-chest surgery, which restricts its use to situations where surgeons already have access to the heart. Dr. Huang’s team aims to create a minimally invasive version that could be inserted through a small tube or catheter. This would make the patch far more practical for widespread clinical use.
The researchers are also collaborating with Xiaoqing (Jade) Wang, an assistant professor of statistics, to develop an AI model that maps immune responses and helps refine dosing strategies for future immunomodulatory treatments. This could optimize patch design and inform additional therapies using other molecules.
Broader Context: Why Heart Regeneration Is Difficult
Heart cells, unlike many cells elsewhere in the body, do not regenerate easily. After childhood, cardiomyocyte turnover slows to a near standstill. When these cells die during a heart attack, they are replaced by scar tissue—not new muscle.
Several strategies have been explored over the years:
- Stem cell injections
- Biomaterial scaffolds
- Growth factor therapies
- Gene therapy approaches
Many of these have shown promising early results but struggled in clinical translation due to safety issues, inconsistent outcomes, or poor tissue integration. The microneedle patch stands out because it focuses on modulating the immune environment, a less invasive and more controllable method that works with the body’s natural healing processes.
By influencing how the immune system behaves immediately after injury, the patch may set the stage for better long-term repair—something regenerative medicine has been striving toward for decades.
Why This Development Matters
If this technology progresses successfully into human clinical trials, it could reshape how heart attack recovery is managed. The potential benefits include:
- Reducing long-term complications
- Improving quality of life for millions of patients
- Lowering medical costs associated with chronic heart failure
- Providing a new framework for localized, biodegradable drug-delivery systems
Beyond the heart, this microneedle-based immunomodulatory approach may inspire treatments for other organs where inflammation plays a strong role in healing.
While early and still limited to preclinical models, the results are undeniably promising. The combination of biomaterials engineering, immunology, and localized drug delivery marks a meaningful step toward effective cardiac regeneration.
Research Paper:
Immunomodulatory microneedle patch for cardiac repair in rodent and porcine models of myocardial infarction
https://doi.org/10.1016/j.celbio.2025.100152