Blocking the TSP-1 and CD47 Interaction Could Help Revive Exhausted T Cells and Improve Cancer Immunotherapy
Researchers at Weill Cornell Medicine have identified a molecular interaction that tumors use to weaken the immune system, and their findings could open the door to more effective cancer treatments. The new study, published in Nature Immunology, reveals how tumors exploit a signaling pathway involving thrombospondin-1 (TSP-1) and CD47 to push cancer-fighting CD8+ T cells into an exhausted, low-function state. Even more promising, the team showed that disrupting this interaction in animal models revived T cell activity and improved tumor control.
This research is important because many cancers manage to evade the immune system not just by hiding, but by actively reprogramming immune cells to stop attacking. The discovery of a new exhaustion-driving pathway gives scientists another therapeutic target—especially for patients who do not respond well to existing treatments like PD-1 checkpoint inhibitors.
Below is a detailed breakdown of what the researchers found, how they tested it, why it matters, and how it could shape the next generation of cancer immunotherapy.
What the Study Found About T Cell Exhaustion
T cell exhaustion happens when immune cells face constant stimulation from chronic infections or tumors. These T cells still recognize their targets, but their ability to kill is severely weakened. This prevents runaway inflammation but also gives cancers an opportunity to grow unchecked.
Earlier research identified PD-1 as a major exhaustion marker, which led to the development of checkpoint-inhibitor therapies. But not all patients benefit from PD-1 drugs, suggesting other mechanisms are also at play. The new study focused on whether the molecule CD47, commonly known for sending a “don’t eat me” signal to evade macrophages, also influences T cell behavior.
The researchers were surprised to discover that CD47 increases dramatically on exhausted T cells, suggesting it plays a more active role in suppressing T cell function than previously understood. When they examined mice lacking CD47, these animals showed slower tumor growth, not because cancer cells were missing CD47, but because the immune cells without CD47 performed better.
This pointed the team toward a different source of suppression: the interaction between TSP-1, a protein produced abundantly by metastatic tumor cells, and CD47 on T cells.
The TSP-1 and CD47 Interaction and Why It Matters
After identifying CD47’s involvement in exhaustion, researchers tested how tumors might activate this pathway. They found that TSP-1 binds to CD47, triggering a signaling cascade inside the T cell that leads to exhaustion. When TSP-1 was absent, T cells showed less exhaustion, reinforcing the idea that this protein–receptor relationship is a critical driver of immune suppression.
This was described by the researchers as a breakthrough moment because eliminating either TSP-1 or CD47 produced similar benefits: stronger immune activity and slower tumor progression. That meant the tumor wasn’t just weakening immune cells through indirect microenvironment effects—it was directly signaling them into dysfunction.
Using the TAX2 Peptide to Block Exhaustion
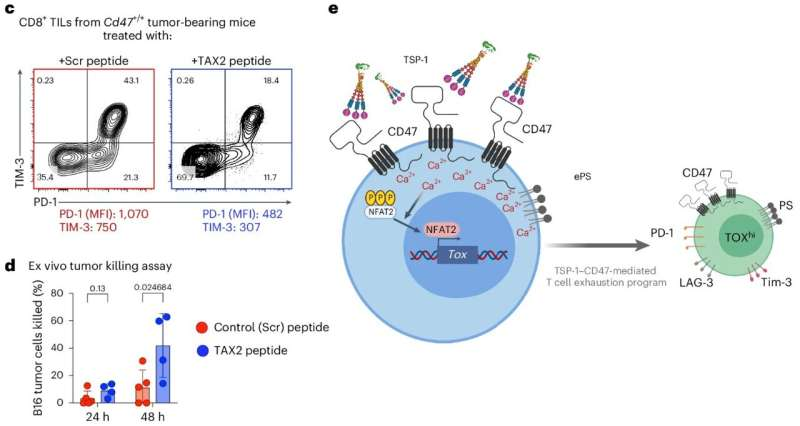
To test whether breaking this interaction could have therapeutic value, the team used a peptide called TAX2, which selectively blocks the binding of TSP-1 to CD47. In mouse models of melanoma and colorectal cancer:
- TAX2 preserved T cell function
- T cells produced more immune-boosting cytokines
- T cells infiltrated tumors more effectively
- Tumor progression slowed significantly
This showed that disrupting the TSP-1–CD47 pathway helps maintain healthy, active T cells inside tumors.
Even more encouraging, TAX2 worked well alongside PD-1 immunotherapy, especially in colorectal tumor models. Existing immunotherapies often become less effective once T cells reach a deeply exhausted state, so preventing exhaustion from developing in the first place could help more patients benefit from treatments already in use.
Why This Discovery Is Important for Cancer Treatment
The study highlights a new mechanism by which tumors suppress immunity. Current immunotherapies focus heavily on checkpoint molecules like PD-1, CTLA-4, TIM-3, and others. But these checkpoint pathways alone do not explain why some patients show resistance or relapse after initial success.
By revealing how tumor-derived TSP-1 can interact with CD47 on T cells—not just cancer cells—the research points to a new way tumors shut down immunity. Blocking this pathway could:
- Prevent early-stage T cell exhaustion
- Improve the longevity and effectiveness of T cell responses
- Enhance the impact of PD-1 and similar checkpoint inhibitors
- Help treat cancers that currently resist immunotherapy
This is of particular significance because the TSP-1–CD47 pathway appears active in both mouse models and human tumors, indicating the mechanism is conserved and likely clinically relevant.
How Tumors Use Exhaustion to Their Advantage
Tumors create an environment that is stressful for immune cells. Continuous exposure to tumor antigens, lack of nutrients, and suppressive molecules gradually push T cells toward dysfunction. Exhausted T cells are characterized by:
- High levels of PD-1, TIM-3, and other checkpoint receptors
- Reduced ability to produce cytokines
- Lower capacity to kill tumor cells
- Expression of transcription factors like TOX, which lock cells into an exhausted state
The newly identified pathway shows how TSP-1 binding to CD47 contributes to this progression. It not only triggers immune checkpoints but also influences the inner signaling and gene expression of the T cell, pushing it deeper into exhaustion.
The Bigger Picture: CD47 Beyond the “Don’t Eat Me” Signal
CD47 is most famous for helping cancer cells avoid being engulfed by macrophages. Many experimental therapies aim to block CD47 on tumors so the immune system can clear them more effectively. However, this study adds a layer of complexity:
- CD47 on T cells, not just cancer cells, plays a major role in immune regulation.
- Tumors take advantage of this by producing TSP-1, strengthening the suppressive effect.
- Therapeutic approaches must therefore consider the dual roles of CD47 to avoid unintended consequences.
A more nuanced strategy—such as selectively blocking the TSP-1 and CD47 interaction without disabling CD47 entirely—may provide safer and more effective outcomes.
What Comes Next for This Line of Research?
The authors plan to investigate:
- Upstream signals that regulate TSP-1 and CD47 expression
- Downstream pathways activated inside T cells once TSP-1 binds CD47
- Potential drugs or biologics that can selectively block this interaction in humans
- Combination therapies using TSP-1–CD47 blockade and PD-1 inhibitors
Blocking this interaction could eventually become a standalone immunotherapy or a booster for existing treatments.
Research Reference
Thrombospondin-1–CD47 signaling contributes to the development of T cell exhaustion in cancer
https://doi.org/10.1038/s41590-025-02321-5