Constipation Drug Shows Unexpected Promise in Slowing Kidney Disease

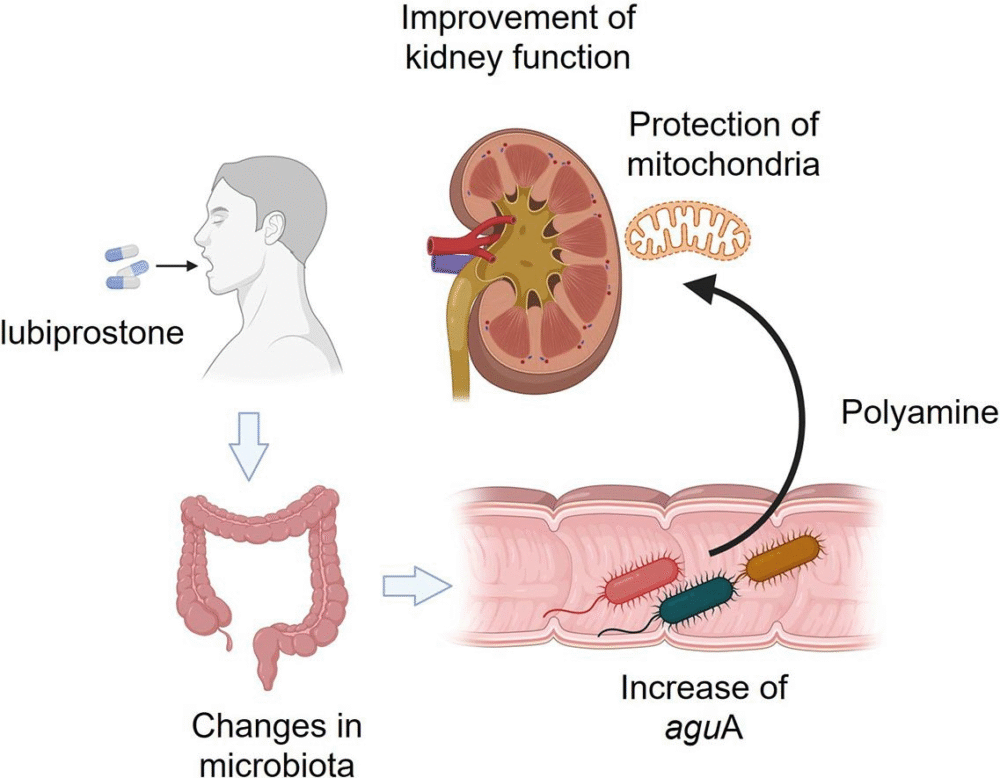
A medication originally developed for constipation is now showing strong potential to protect kidney function in people with chronic kidney disease (CKD). Researchers at Tohoku University Graduate School of Medicine in Japan led a Phase II multicenter clinical trial that revealed surprising results. The study, published in Science Advances on August 29, 2025, is the first to demonstrate that lubiprostone may help slow kidney decline by improving gut health and mitochondrial performance.
Why This Matters
CKD is a serious global health issue. Millions of people worldwide experience a gradual loss of kidney function that often ends with the need for dialysis or a transplant. Until now, there has been no approved drug that directly improves kidney performance. Standard treatments mainly focus on controlling blood pressure, reducing uremic toxins, and managing symptoms.
This trial introduces a completely different strategy: addressing constipation and its impact on the gut microbiota, which in turn influences kidney health.
Credit: Shun Watanabe
Trial Design and Participants
The LUBI-CKD trial enrolled 118 patients across nine medical centers in Japan between 2016 and 2019. Participants had moderate to severe CKD (stage IIIb–IV), with estimated glomerular filtration rates (eGFR) between 25 and 45 mL/min/1.73 m².
The trial was randomized, double-blind, and placebo-controlled, lasting 24 weeks. Patients were divided into three groups:
- Placebo
- 8 µg lubiprostone daily
- 16 µg lubiprostone daily
The primary endpoint was a change in indoxyl sulfate, a uremic toxin linked to CKD progression. Secondary endpoints included changes in eGFR, blood urea nitrogen (BUN), and proteinuria.
Results and Findings
- The primary endpoint (indoxyl sulfate levels) did not show a significant change with lubiprostone.
- However, the secondary outcomes revealed something far more important: patients taking 16 µg lubiprostone daily experienced a statistically significant preservation of kidney function, measured by slower eGFR decline compared with placebo (P = 0.0457).
- The protective effect appeared dose-dependent, as both the 8 µg and 16 µg groups showed less kidney decline than the placebo group.
- This makes lubiprostone the first drug to demonstrate a direct renoprotective effect in a CKD population through gut-targeted therapy.
How Does It Work?
The research team looked deeper into the mechanism using multiomics analysis. They discovered that lubiprostone changed the gut microbiota in a way that activated the agmatine (aguA) pathway, boosting production of spermidine.
Spermidine is important because it:
- Improves mitochondrial function
- Reduces inflammation
- Supports cellular energy and repair
This gut–mitochondria–kidney link appears to be the key to why lubiprostone slowed kidney decline. By altering the gut microbiome, the drug indirectly strengthened mitochondrial health, which helped protect the kidneys.
Safety and Side Effects
Like most laxatives, lubiprostone did cause some gastrointestinal side effects. The most common was diarrhea, affecting 12.1% of patients at 8 µg and 16% at 16 µg. Importantly, the discontinuation rates were similar between lubiprostone and placebo groups, suggesting the side effects were manageable.
Limitations of the Study
While the findings are exciting, there are important caveats:
- The trial had a small sample size of 118 patients.
- It lasted only 24 weeks, which is relatively short for a disease like CKD.
- All participants were from Japan, so it’s not yet clear if results will apply to more diverse populations.
- The renoprotective effect was technically a secondary endpoint, not the main study goal.
Because of these factors, the results should be seen as promising but preliminary.
What’s Next?
The Tohoku University team has announced plans for a Phase III clinical trial with a larger and more diverse patient population. They also intend to explore biomarkers that could predict which patients will respond best to the treatment.
If confirmed, lubiprostone could represent a new therapeutic approach to CKD — one that targets the gut microbiome and mitochondrial function rather than focusing only on toxin control. This strategy may even open doors for treating other conditions tied to mitochondrial dysfunction.
Conclusion
This trial highlights how a drug initially designed for constipation may reshape the treatment of CKD. By focusing on the gut–kidney connection, lubiprostone has shown it can slow kidney decline, making it one of the most exciting new leads in kidney medicine.
TL;DR
A Phase II Japanese trial found that lubiprostone, a constipation drug, slowed kidney decline in CKD patients by altering gut bacteria and boosting mitochondrial function. Results were dose-dependent, with side effects mainly limited to mild diarrhea. Larger trials are planned.