Cooling-Activated Implantable Drug Delivery Could Change How Targeted Pain Relief Is Managed
Researchers at Vanderbilt University have developed a novel implantable drug delivery system that releases pain-relief medication when exposed to cooling, offering a potentially safer and more patient-controlled alternative to conventional pain treatments. The technology relies on a temperature-responsive hydrogel device that can be triggered using something as simple as an ice pack, eliminating the need for electronics, batteries, or specialized external equipment.
The work is led by Leon Bellan, an associate professor of mechanical and biomedical engineering at Vanderbilt, along with his research team. Their findings were published in the peer-reviewed journal ACS Biomaterials Science & Engineering under the title Cooling-Triggered Release of Celecoxib from Implantable Alginate-Soluplus Composite Devices.
Why a New Approach to Pain Relief Is Needed
Pain management remains one of the most challenging areas of modern medicine. Today, on-demand pain control is still largely dominated by opioid medications, which are effective but come with serious risks. Opioids are highly addictive, and opioid misuse continues to cause a significant number of deaths every year in the United States alone.
Because of this, researchers and clinicians are increasingly looking toward nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and celecoxib. These drugs are non-addictive, effective against pain and inflammation, and well understood from a clinical perspective. However, NSAIDs also have limitations, particularly when taken orally or systemically over long periods. High systemic doses can lead to gastrointestinal, cardiovascular, and kidney-related side effects.
One promising solution is local drug delivery, where medication is released directly at the site where pain occurs. This approach can reduce the total amount of drug needed and limit side effects throughout the rest of the body. The missing piece, until now, has been a simple and reliable on-demand release mechanism that patients can control themselves.
How the Cooling-Triggered Device Works
The Vanderbilt team’s solution is an implantable drug depot, roughly the size of a watch battery, designed to be placed beneath the skin near a site of chronic or post-surgical pain. Inside the device is a thermoresponsive hydrogel composite made from alginate and Soluplus, two materials commonly used in biomedical and pharmaceutical applications.
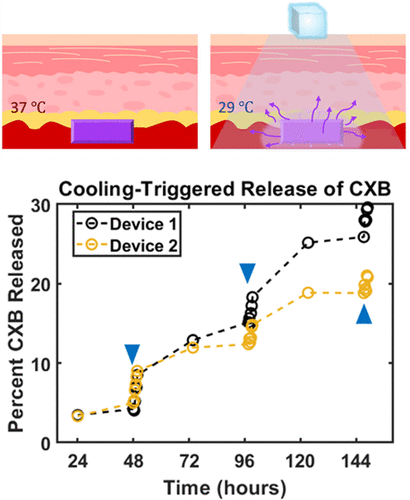
At normal body temperature, around 37°C, the hydrogel remains stable and holds the drug in place. When the temperature around the implant drops—typically to about 29°C—the material undergoes a gel-to-liquid transition. This physical change allows the encapsulated drug to be released rapidly into the surrounding tissue.
Importantly, this temperature drop does not require extreme cold. A simple ice pack applied externally to the skin above the implant is enough to trigger the release. Once cooling stops and the tissue returns to normal temperature, the hydrogel stabilizes again, slowing drug release until the next cooling cycle.
The Drug Used in the Study: Celecoxib
In their experiments, the researchers focused on celecoxib, a widely used NSAID commonly prescribed for arthritis and inflammatory pain. Celecoxib is particularly well suited for local delivery because it effectively reduces inflammation while minimizing gastrointestinal side effects compared to some older NSAIDs.
By embedding celecoxib within the alginate-Soluplus composite, the team demonstrated that the drug could remain sequestered within the implant for extended periods and be released only when cooling was applied. This design allows patients to control when pain relief occurs, rather than relying on fixed dosing schedules.
Key Findings From the Research
Laboratory testing showed that cooling the implant resulted in a dramatic increase in drug release, with rates rising by as much as 40 times compared to release at normal body temperature. Just as importantly, the system was shown to work over multiple cooling cycles, meaning it does not empty its entire drug supply after a single activation.
The researchers validated the device across several experimental models, including:
- Simulated body fluids
- Cell culture systems
- Ex vivo tissue samples
- Animal wound models
These progressively complex tests demonstrated that the cooling-triggered mechanism remains effective in biologically realistic environments.
Why Cooling Is a Big Advantage
Many previously developed stimulus-responsive drug delivery systems rely on heat, light, ultrasound, or electrical signals. While effective in controlled settings, these methods often require external power sources, specialized equipment, or clinical supervision.
Cooling stands out because it is passive, accessible, and familiar. Patients already use ice packs for pain and inflammation, so integrating cooling into a drug delivery strategy feels intuitive rather than invasive. There is no need for batteries, wires, wireless controllers, or implanted electronics, which also reduces the risk of mechanical failure.
From a safety standpoint, cooling also avoids the tissue damage risks that can accompany excessive heating or focused energy delivery.
Potential Applications Beyond Pain Relief
While the current study focuses on pain management, the broader implications are significant. A cooling-triggered implant could potentially be adapted to deliver medications for other conditions where localized, on-demand dosing is beneficial.
Possible future applications include anti-inflammatory treatments for joint diseases, post-surgical recovery support, localized cancer therapies, or even infection control at surgical sites. The core concept—using temperature as a trigger—could also be adapted to other drugs and hydrogel formulations.
Challenges and Next Steps
Although the results are promising, the technology is still in the preclinical research stage. Further studies will be needed to evaluate long-term safety, implant stability, immune responses, and precise dosing control in humans. Regulatory approval and clinical trials would be required before such devices could be widely used in medical practice.
That said, the simplicity of the design and the use of well-known materials may help streamline future development and approval processes.
A Step Toward Patient-Controlled Medicine
This cooling-activated implant represents a broader trend in medicine toward patient-controlled therapies. Instead of relying solely on scheduled dosing or clinic-based interventions, patients gain the ability to manage symptoms in real time, using a method that is both intuitive and low-tech.
By combining biomaterials engineering, drug delivery science, and a deep understanding of patient needs, the Vanderbilt team has introduced a compelling new approach to localized pain relief—one that could help reduce reliance on opioids while improving quality of life.
Research paper:
https://doi.org/10.1021/acsbiomaterials.5c00867