Engineered Extracellular Vesicles Show Promise in Halting Lung Cancer Growth by Silencing a Key Protein

In a new study from the University of Missouri, scientists have taken an exciting step toward more targeted treatments for lung cancer using something most people have never heard of — extracellular vesicles (EVs). These microscopic, bubble-like structures naturally released by cells may hold the key to stopping cancer growth without the harsh side effects of traditional therapies like chemotherapy.
At the center of this breakthrough is Akhil Srivastava, an assistant professor at the University of Missouri School of Medicine, and his research team. Their findings, published in the journal Molecular Therapy Oncology in 2025, show that by modifying the molecular contents of these vesicles, tumors can actually shrink instead of spreading.
What Are Extracellular Vesicles and Why They Matter
Extracellular vesicles are tiny communication packages that cells send out to exchange information. They are so small that thousands of them could fit across the width of a single human hair. Every cell in the body produces these vesicles — whether healthy or cancerous.
EVs act as cellular messengers, carrying proteins, genetic material, and other molecules between cells. When they come from healthy cells, they generally promote normal functioning and healing. However, EVs released from cancer cells tend to do the opposite — they spread harmful signals that encourage tumor growth, metastasis, and even resistance to existing cancer treatments.
Because EVs can move freely through the bloodstream and tissues, they serve as both potential biomarkers and drug delivery vehicles. Scientists have been studying them for years, but Srivastava’s team has now found a way to manipulate these vesicles to actually turn them against cancer.
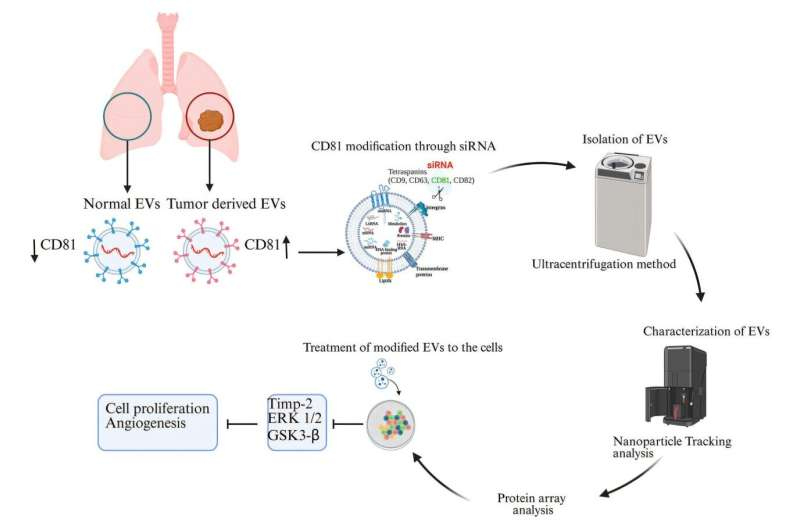
The Role of CD81 in Lung Cancer
In their study, the researchers identified a specific protein called CD81 that seems to play a critical role in how cancer spreads through EVs. CD81 belongs to a family of proteins known as tetraspanins, which are often found on the surface of extracellular vesicles.
When Srivastava and his team compared EVs from healthy lung cells to those from lung cancer cells, they discovered that the cancer-derived vesicles contained much higher levels of CD81. This suggested that CD81 might be helping the tumor cells communicate and grow.
To test this theory, they used small interfering RNA (siRNA) — short sequences of genetic material that can “silence” or block the production of specific proteins. By introducing siRNA into lung cancer cells, the researchers successfully stopped these cells from producing CD81.
The result was striking: the cancer cells began producing EVs that no longer supported tumor growth. Instead, these modified vesicles helped shrink tumors in preclinical experiments.
How Silencing CD81 Helped Stop Tumor Growth
The idea of silencing CD81 is simple in concept but powerful in effect. Normally, lung cancer cells send out EVs filled with instructions that help tumors expand and invade nearby tissues. But when CD81 is silenced, the EVs lose this ability to “communicate” those harmful instructions.
When the researchers tested this approach in preclinical models, the results confirmed their theory — tumors grew more slowly, and in many cases, even shrunk. This demonstrated that CD81 isn’t just a passive marker of cancer but an active player in how the disease progresses.
This finding makes CD81 a strong candidate as both a diagnostic biomarker (to help detect aggressive cancers) and a therapeutic target (to block or reverse tumor-promoting effects).
The Potential of EVs as Cancer Treatments
Srivastava’s work doesn’t stop at simply silencing harmful EVs. His ultimate goal is to engineer these vesicles to deliver treatment directly to cancer cells, much like labeling a package for a specific address.
The same study explored how EVs could be used as delivery vehicles for siRNA therapies. By loading small pieces of siRNA into modified EVs, the team managed to make the vesicles carry genetic instructions designed to kill cancer cells — while leaving healthy cells largely unharmed.
This concept is particularly appealing because it tackles one of the biggest problems in cancer treatment today: selectivity.
- Chemotherapy kills both healthy and cancerous cells, often causing severe side effects.
- Immunotherapy, while promising, doesn’t work for all patients and can be prohibitively expensive.
A therapy based on EVs could potentially solve both problems by being highly specific, less toxic, and more adaptable.
Challenges and the Road Ahead
Although the findings are promising, this research is still in its preclinical stage. That means it’s been tested in cell cultures and animal models, but not yet in human trials. Translating this kind of therapy into human medicine involves major challenges:
- Manufacturing at scale – Producing enough engineered EVs for human treatment is complex.
- Targeting accuracy – Ensuring the EVs reach the right cells in the body remains a key technical hurdle.
- Safety testing – Because EVs are also used by healthy cells for normal communication, scientists must ensure that manipulating them doesn’t disrupt other body systems.
- Regulatory approval – New forms of gene and vesicle-based therapies will require rigorous testing before clinical use.
Despite these hurdles, Srivastava’s findings mark a meaningful step forward. By proving that EVs can be reprogrammed to fight cancer, his work opens the door to a new class of precision treatments that may one day work alongside — or even replace — chemotherapy and immunotherapy for certain cancers.
A Broader Look at EV Research in Cancer
This study fits into a growing global effort to understand how EVs influence cancer. In recent years, researchers have discovered that cancer-derived EVs can:
- Prepare pre-metastatic niches — environments in distant organs that make it easier for cancer to spread.
- Suppress immune responses, helping tumors evade detection.
- Promote angiogenesis, or the formation of new blood vessels, which supply the tumor with oxygen and nutrients.
Other studies have shown that circulating EVs can serve as liquid biopsy biomarkers, allowing doctors to detect cancer or monitor treatment response from a simple blood sample.
Interestingly, EVs have also been studied in connection with immunotherapy outcomes. Some studies found that higher levels of CD81-positive EVs in the bloodstream could correlate with better responses to immune checkpoint inhibitors in lung cancer patients. This indicates that CD81 is a versatile molecule that could be both a marker of disease progression and a therapeutic tool, depending on how it’s used.
Why This Matters for the Future of Lung Cancer Treatment
Lung cancer remains the leading cause of cancer-related deaths worldwide, largely because it is often diagnosed late and can be resistant to current treatments. New, targeted therapies are desperately needed.
The work from the University of Missouri brings hope that future treatments could become more precise, less toxic, and more effective by using the body’s own communication tools — extracellular vesicles.
Instead of fighting cancer with a blunt weapon, scientists could use engineered EVs as precision instruments, reprogramming them to carry therapeutic messages that attack tumors directly.
This kind of research also highlights a broader shift in medicine: moving from traditional drug chemistry toward biological engineering, where cells, genes, and natural biological particles become part of the treatment itself.
Reference
Research Paper: Perturbed CD81 in Lung-Cancer-Derived Extracellular Vesicles Modifies Its Function in Cancer Pathophysiology – Molecular Therapy Oncology (2025)