Engineered Protein Complex Could Help Immunotherapies Target Hard-to-Treat Neuroblastoma
Researchers from Children’s Hospital of Philadelphia (CHOP) have reported a promising new strategy that could make cancer immunotherapies effective against some of the most difficult tumors to treat, including neuroblastoma. The study describes an engineered protein complex designed to help the immune system recognize tumors that usually remain hidden, a major obstacle in modern cancer therapy. The findings were published in Science Advances in early 2026.
Immunotherapies have transformed cancer treatment over the past decade, but they still depend on one critical requirement: tumor cells must be visible to the immune system. Many cancers fail this test. These tumors, often referred to as “cold” tumors, do not trigger strong immune responses and therefore evade immune-based treatments. Neuroblastoma, a childhood cancer that arises from immature nerve cells, is considered one of the coldest tumors known.
The CHOP research team, led by Nikolaos G. Sgourakis, focused on addressing this invisibility problem at its biological roots. Their work centers on restoring the tumor’s ability to display molecular signals that immune cells rely on to identify cancerous cells.
Why Cold Tumors Are So Hard to Treat
For the immune system to attack cancer, tumor cells must present small protein fragments, known as antigens, on their surface. These antigens are displayed using Class I human leukocyte antigen (HLA-I) proteins, which function much like barcodes. When immune cells, especially T cells, scan the body, they use these barcodes to distinguish normal cells from infected or malignant ones.
Many tumors, including neuroblastoma, reduce or completely suppress HLA-I expression on their surface. This loss of HLA-I is a well-known mechanism of immune evasion. Without these markers, T cells struggle to recognize tumor cells, making immunotherapies far less effective.
Neuroblastoma poses an additional challenge. These tumors often have low mutation rates, produce very few recognizable antigens, and show minimal T-cell infiltration. Together, these features make traditional immunotherapy strategies largely ineffective.
A New Approach: Restoring Antigen Presentation
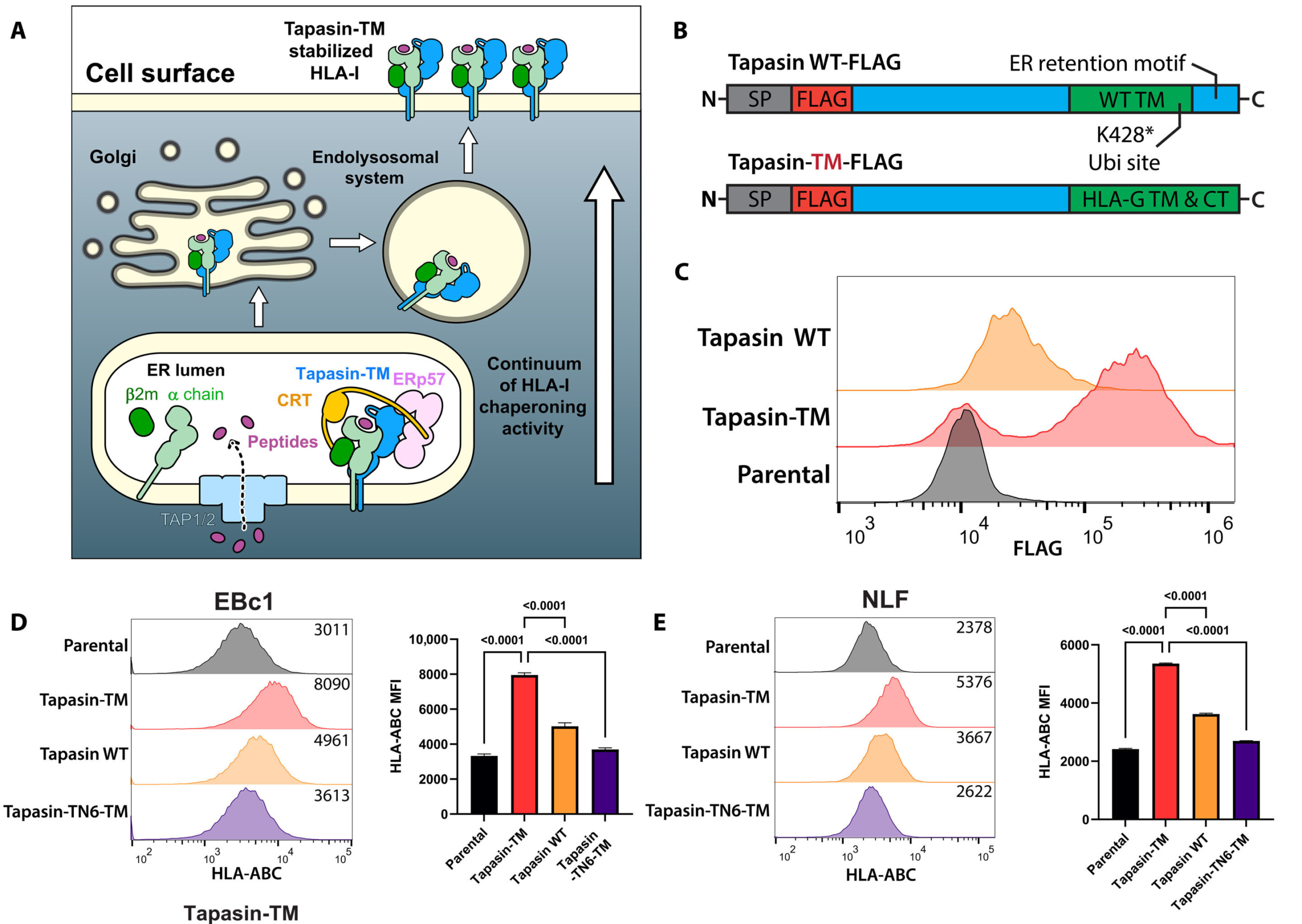
To tackle this problem, the researchers developed an engineered protein system known as HLA-Shuttle. Rather than directly attacking tumor cells, HLA-Shuttle works by manipulating the cellular machinery responsible for producing and transporting HLA-I molecules.
HLA-Shuttle acts as a chaperone-like complex, guiding HLA-I proteins through the cell and restoring their presence on the tumor cell surface. By doing so, it effectively forces cold tumor cells to display antigens again, making them visible to immune surveillance.
This approach is different from many existing immunotherapies. Instead of boosting immune activity or adding external immune cells, HLA-Shuttle focuses on correcting the tumor’s ability to present antigens in the first place.
What the Researchers Found
In laboratory experiments using neuroblastoma cell lines, HLA-Shuttle successfully restored HLA-I surface expression in cells that previously showed very low levels. This restoration reactivated the antigen presentation pathway, allowing immune recognition mechanisms to function again.
One of the most striking outcomes of the study was the discovery of 180 distinct peptides mapped to 30 different genes that became visible once HLA-I expression was restored. Many of these peptides were previously unknown or understudied in the context of neuroblastoma.
This finding is significant because it expands the pool of potential therapeutic targets. These newly revealed peptides could be used not only for immunotherapies such as T-cell–based treatments but also for other precision medicine approaches.
Importantly, the researchers emphasized that HLA-Shuttle is not limited to enhancing immune recognition alone. By bringing hidden proteins to the cell surface, it also serves as a powerful tool for discovering new targets that were previously inaccessible.
Current Stage and Next Steps
So far, the work has been conducted outside the body, using cultured tumor cells. While the results are encouraging, the next major challenge is translating this system into a form that can be safely delivered in living organisms.
Future research will focus on optimizing delivery methods, evaluating safety, and testing how well HLA-Shuttle works in more complex biological systems. Researchers at CHOP and the University of Pennsylvania are collaborating to determine which tumor types and treatment combinations could benefit most from this technology.
Although the initial focus has been neuroblastoma, the researchers believe the approach could also be applied to other cold tumors, including pancreatic, ovarian, and prostate cancers, as well as various sarcomas. There is also interest in testing the system in tumors that are already somewhat immunogenic, sometimes called “warmer” tumors, to further expand the range of possible therapeutic targets.
Why This Matters for Immunotherapy
The effectiveness of immunotherapy depends heavily on antigen visibility. Many existing treatments fail not because the immune system lacks power, but because it lacks information. By restoring antigen presentation, HLA-Shuttle addresses a fundamental limitation that has hindered progress against cold tumors for years.
If successfully translated into clinical use, this strategy could help turn tumors once considered untreatable into viable targets for immune-based therapies. It may also reduce the need for highly aggressive treatments that cause significant side effects, especially in pediatric cancers like neuroblastoma.
Understanding Neuroblastoma and Immune Evasion
Neuroblastoma is the most common cancer in infants and one of the leading causes of cancer-related death in young children. High-risk cases often resist conventional therapies, including chemotherapy and radiation.
One reason neuroblastoma has been so resistant to immunotherapy is its ability to remain immunologically silent. Unlike cancers with many mutations, neuroblastoma cells often appear deceptively normal to immune cells. Strategies like HLA-Shuttle aim to change that perception by revealing what the immune system has been missing.
Broader Implications Beyond Neuroblastoma
The principles behind HLA-Shuttle extend beyond a single cancer type. Loss of HLA-I expression is a common feature in many advanced cancers. Restoring this pathway could enhance the effectiveness of a wide range of immune-based treatments, including cancer vaccines and adoptive T-cell therapies.
Additionally, the ability to uncover hidden antigens could accelerate cancer research by providing new insights into tumor biology and immune escape mechanisms.
Looking Ahead
While much work remains before HLA-Shuttle can be tested in patients, the study represents a meaningful step forward in addressing one of cancer immunotherapy’s biggest challenges. By focusing on visibility rather than force, the approach opens new possibilities for treating tumors that have long resisted immune attack.
As research progresses, HLA-Shuttle may help redefine how scientists think about targeting cold tumors and expanding the reach of immunotherapy to cancers that were previously considered out of reach.
Research paper:
https://www.science.org/doi/10.1126/sciadv.aeb0821