Fertility Treatments in Mice Linked to a Higher Rate of DNA Mutations Compared to Natural Conception
A new study from researchers at The Jackson Laboratory has brought forward an interesting finding: mice conceived through common fertility treatments show a modest but measurable increase in DNA mutations compared to mice conceived naturally. The research, published in Genome Research, explores how assisted reproductive technologies (ART) such as hormone stimulation, in vitro fertilization (IVF), and embryo transfer may subtly influence the genetic outcomes of offspring. While the study focuses purely on mice and avoids drawing direct conclusions about humans, the results raise important questions about the biological effects of fertility treatments.
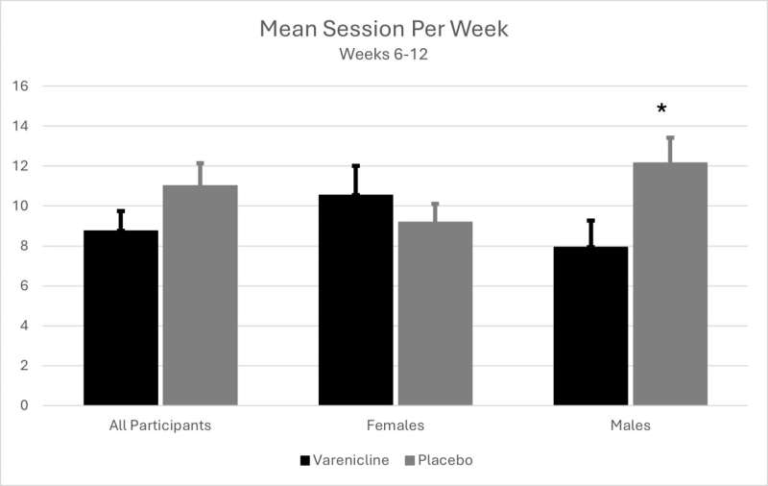
The experiment involved comparing whole-genome sequences of mice born naturally with those produced through a standard ART procedure. The ART protocol used in the study included hormone-induced ovarian stimulation, collection and fertilization of eggs in the laboratory, embryo culture, and transfer of embryos into surrogate mothers. After sequencing the genomes of both groups, the researchers found that the mice conceived through ART had about 30% more new single-nucleotide mutations—tiny changes in DNA involving a single letter, also known as point mutations.
These single-nucleotide variants (SNVs) arise when DNA replicates incorrectly, and although the vast majority are harmless, they still represent meaningful biological signals. In this study, the mutations appeared evenly scattered across the genome, rather than concentrated in particular genes or regions. The timing of when these mutations emerged—during early embryo development—also appeared similar between naturally conceived mice and ART-conceived mice. This suggests that the ART process does not alter when mutations happen, only the overall likelihood that new DNA changes will occur.
Despite the increase, the researchers emphasize that the absolute risk remains very low. Only a small portion of new mutations—estimated at less than 2%—is expected to have any harmful effect. Based on the mutation rate observed, the researchers estimate that in roughly every 50 mice conceived through IVF, there may be one additional potentially harmful mutation compared to natural conception. Considering the mouse genome contains about 2.7 billion DNA letters, this number is extremely small in context.
One interesting comparison made by the scientists is that a similar increase in mutation rate could also occur in mice if the father were 30 weeks older, since paternal age is a known driver of mutation accumulation in mammals. This helps contextualize the size of the effect—significant enough to measure but still modest.
Possible Biological Explanations
The study does not pinpoint a single cause behind the increased mutation rate. Instead, several possible contributors within the ART process are highlighted:
- Hormone-induced ovarian stimulation is one potential source. These hormones push eggs to re-enter meiosis, a complex cell division stage known to be prone to minor replication errors.
- Laboratory embryo culture, which subjects embryos to different chemical environments than they would encounter naturally, could introduce mild stress on developing cells.
- Physical handling of embryos, which includes transferring embryos between lab environments or into surrogate mothers, may also influence DNA repair mechanisms.
Any of these steps—or a combination of them—could slightly alter the stability of the developing embryo’s DNA. Further research is needed to understand exactly where the changes originate.
What This Means for Humans
Importantly, the findings cannot be directly applied to people. Mice and humans differ significantly in reproductive biology, including how their reproductive cycles work—mice, for example, do not menstruate. Additionally, human fertility treatments use different culture media, hormonal regimens, and clinical protocols.
Another complication is that human patients who undergo IVF often have a range of underlying fertility issues or environmental exposures that already influence genetic outcomes. These confounding factors would make it difficult to isolate the effect of ART on mutation rates in humans.
However, the study does reinforce the idea that it is worth examining human fertility treatments with more detail. Prior research has shown that ART can influence epigenetics—chemical markers on DNA—even when the DNA sequence itself remains unchanged. This new study introduces the possibility, at least in mice, that ART might also have a small effect on the DNA sequence itself.
The researchers encourage further human-based studies, ideally using whole-genome sequencing of parents and IVF-conceived children, to explore whether similar trends occur in clinical settings. For now, no evidence exists showing a comparable effect in humans, and existing clinical data does not point to a significant rise in harmful mutations among IVF-conceived individuals.
A Closer Look at Single-Nucleotide Mutations
Because this study focuses primarily on single-nucleotide variants, it’s helpful to understand what these mutations represent. DNA is made up of four chemical bases—A, T, C, and G—that function like letters in a long instruction manual. A single-nucleotide mutation changes just one of these letters. Most of the time, such changes are neutral: they occur in noncoding regions, or they do not meaningfully alter how a gene functions.
Scientists estimate that organisms naturally acquire dozens of new point mutations in each generation, even without any environmental stressors or medical interventions. These mutations are part of normal evolutionary processes. The key observation in this study is not that ART introduces harmful mutations, but that it slightly increases the rate at which random mutations arise.
Why These Findings Matter
As assisted reproduction becomes increasingly common worldwide, understanding the full biological impact of these technologies is essential. Even if the risks remain extremely small, studies like this help ensure that fertility treatments continue to improve in safety and effectiveness.
This research also highlights the importance of studying early embryonic development under different conditions. Because embryos grow and divide rapidly, they are particularly sensitive to environmental and hormonal influences. Small insights into how laboratory conditions affect genetic stability could help optimize IVF processes not just for mice but potentially for humans as well.
What Future Research Might Explore
Several scientific directions could help build on these findings:
- Identifying which specific step in ART contributes most to increased mutation rates.
- Testing whether different culture media or hormonal protocols reduce mutation occurrences.
- Investigating whether the same trend appears in different strains of mice or other mammals.
- Conducting long-term studies on the health outcomes of ART-conceived mice.
- Running advanced genomic studies on human IVF-conceived children to look for subtle mutation trends.
Building a deeper understanding in these areas could help guide clinical improvements and maintain the safety profile of fertility treatments.
Reference to the Research Paper
Modest increase in the de novo single nucleotide mutation rate in house mice born by assisted reproduction (Genome Research, 2025)
https://genome.cshlp.org/content/early/2025/11/12/gr.281180.125.full.pdf+html