GADD45A Protein Shows Promise in Preventing Harmful Heart Remodeling

Researchers have discovered that the protein GADD45A plays a protective role in the heart, helping to guard against dangerous stress responses that can eventually lead to heart failure. This finding, published in the journal Cellular and Molecular Life Sciences, adds an important new piece to our understanding of how the heart adapts to pressure and stress, and why conditions like type 2 diabetes can significantly increase the risk of cardiovascular disease.
Why the Heart Thickens Under Stress
When the heart is forced to work harder, its muscular walls may thicken in response. This process is known as cardiac hypertrophy. In the short term, this is an adaptive change: the thickened wall allows the heart to handle increased workload and maintain proper function. In many cases, the process can reverse itself if the stress subsides.
But when stress persists over the long term, this thickening shifts into what doctors call pathological hypertrophy. In this stage, instead of protecting the heart, the changes start to damage it. The ventricles may enlarge abnormally, heart muscle performance declines, and the person becomes more vulnerable to conditions like heart failure.
Why People With Diabetes Are More at Risk
Individuals with type 2 diabetes (T2D) are especially vulnerable to this dangerous form of hypertrophy. This higher risk is due to a combination of factors that often overlap with diabetes, including high blood pressure, obesity, and coronary artery disease. Together, these conditions make the heart more likely to develop lasting structural changes, leading to poor outcomes over time.
Introducing GADD45A
The newly highlighted player in this process is GADD45A, short for growth arrest and DNA damage inducible 45A. This protein has been known to scientists for some time as a multifunctional factor involved in stress signaling and cell damage repair. It has also been studied in cancer research, metabolism, and inflammatory diseases.
In the heart, however, its role was less clear until now. The team behind this study, led by Professor Manuel Vázquez-Carrera and Associate Professor Xavier Palomer from the University of Barcelona’s Faculty of Pharmacy and Food Sciences, examined GADD45A in both animal models and human cardiac cells. Their work was supported by the Institute of Biomedicine of the UB (IBUB), the Sant Joan de Déu Research Institute (IRSJD), and the CIBERDEM research network. The first author of the paper is Adel Rostami.
What the Study Found
The researchers looked at the effects of either removing GADD45A or boosting its presence in the heart.
Findings in Mice
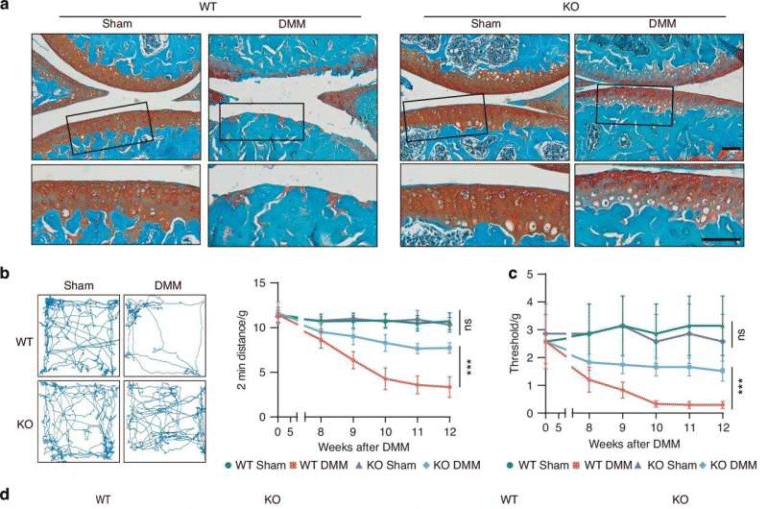
- Mice lacking the GADD45A gene developed significant cardiac fibrosis, inflammation, and apoptosis (programmed cell death).
- These harmful changes were tied to hyperactivation of well-known transcription factors that drive damage: AP-1, NF-κB, and STAT3.
- Mice without GADD45A showed clear signs of cardiac hypertrophy, which worsened the structure and performance of the heart.
Findings in Human Heart Cells
- In human AC16 cardiomyocytes, boosting GADD45A (overexpression) offered protection.
- When these cells were exposed to TNF-α, a strong inflammatory trigger, overexpression of GADD45A partly prevented the usual inflammatory and fibrotic response.
- This demonstrates that GADD45A can act as a brake against damaging signaling in human heart cells as well.
Big Picture
Taken together, the results suggest that GADD45A helps prevent inflammation, fibrosis, and cell death in the heart. When it is absent or reduced, the heart is left vulnerable to damaging remodeling. When it is boosted, the heart appears more resilient under stress.
Why Fibrosis and Inflammation Matter
One of the most critical aspects of pathological hypertrophy is fibrosis, where scar-like tissue builds up inside the heart muscle. Fibrosis makes the heart stiff, reduces its efficiency, and worsens outcomes for patients.
Inflammation is another key driver. Chronic inflammatory signaling not only promotes fibrosis but also accelerates the deterioration of cardiac function. By limiting both fibrosis and inflammation, GADD45A offers a natural line of defense against heart failure progression.
GADD45A Beyond the Heart
This isn’t the first time GADD45A has attracted scientific attention.
- Cancer Research: GADD45A is known as a tumor suppressor, helping prevent the uncontrolled cell growth that characterizes cancer.
- Metabolic Regulation: It has been linked to balancing catabolic and anabolic pathways in metabolism.
- Inflammatory Diseases: Studies have shown that GADD45A plays a role in controlling oxidative stress, inflammation, and fibrosis in other tissues as well.
- Diabetes and Obesity: Some earlier studies suggest that modulating GADD45A could help prevent obesity and diabetes by regulating fat and energy metabolism.
The current research adds cardiac protection to this growing list of roles.
Mechanisms at Work
The harmful effects observed when GADD45A is deleted seem to come from overactivation of certain transcription factors:
- AP-1 (Activator Protein-1)
- NF-κB (Nuclear Factor-κB)
- STAT3 (Signal Transducer and Activator of Transcription 3)
These proteins control the expression of genes involved in inflammation and fibrosis. Without GADD45A to keep them in check, the pathways run unchecked, leading to damage.
In contrast, overexpression of GADD45A tones down the response, preserving heart structure and function.
Implications for Therapy
If these findings translate to humans, boosting GADD45A activity could become a therapeutic strategy to slow or even prevent the progression from adaptive hypertrophy to full-blown heart failure.
However, this is still an early-stage discovery. Much more research is needed before therapies can be developed. Key questions remain, such as:
- How can GADD45A be safely boosted in patients?
- Would therapies work equally well in people with and without diabetes?
- Are there risks involved, given GADD45A’s roles in other tissues?
Still, the results are promising and open up a new line of investigation for treating heart disease.
A Closer Look: Cardiac Hypertrophy
Since hypertrophy is central to this research, here’s a brief overview of what it means in everyday terms.
- Adaptive Hypertrophy: This happens, for example, in athletes. The heart becomes stronger and more efficient to handle increased demand. This type of hypertrophy is usually healthy and reversible.
- Pathological Hypertrophy: This occurs in chronic conditions such as high blood pressure or diabetes. Unlike the athlete’s heart, this change is harmful, leading to stiff walls, larger ventricles, and weaker function.
- Transition Point: The real danger lies in when the adaptive process crosses into the pathological stage. That’s where proteins like GADD45A may decide the outcome.
Why This Matters
Heart failure remains a leading cause of death worldwide. While treatments exist—like ACE inhibitors, beta-blockers, and lifestyle management—the ability to directly influence the remodeling process inside the heart is limited.
A discovery like this highlights the possibility of molecular therapies that act directly on the signals driving damage. If GADD45A can be safely harnessed, it could complement existing treatments and give doctors new tools to help patients with diabetes and other heart risk factors.
The Research Team
The study represents a collaboration between multiple institutions:
- University of Barcelona (UB), Faculty of Pharmacy and Food Sciences
- Institute of Biomedicine of UB (IBUB)
- Sant Joan de Déu Research Institute (IRSJD)
- CIBERDEM (Diabetes and Associated Metabolic Diseases research network)
Led by Prof. Manuel Vázquez-Carrera and Assoc. Prof. Xavier Palomer, the team worked with first author Adel Rostami, along with Javier Pizarro-Delgado, Lucía Peña, Mònica Zamora, Marta Montori-Grau, Emma Barroso, Brenda Valenzuela-Alcaraz, Fàtima Crispi, Jesús M. Salvador, Raquel García, María A. Hurlé, and Francisco Nistal.
Conclusion
The discovery of GADD45A’s protective role against cardiac remodeling is a significant step forward. By keeping inflammation and fibrosis under control, this protein may help preserve heart function under stress, especially in people with diabetes. While the path to therapy is long, the research opens exciting new possibilities for tackling heart disease at the molecular level.