Gene Variant Linked to Increased Brain Inflammation and Higher CTE Risk in People With Repetitive Head Impacts

A new study has taken a major step toward explaining why some people with similar histories of repetitive head impacts end up with very different brain outcomes. While activities like contact sports, military service, and repeated concussions are known contributors to Chronic Traumatic Encephalopathy (CTE), this research points to a specific genetic factor that may dramatically increase vulnerability: a variation in the TMEM106B gene.
Credit: Boston University
Researchers from Boston University’s CTE Center have identified how this gene influences the brain’s inflammatory response and how it may elevate the risk of developing more severe CTE, dementia, and additional neurodegenerative markers. The findings are published in the journal Acta Neuropathologica.
What the Study Looked At
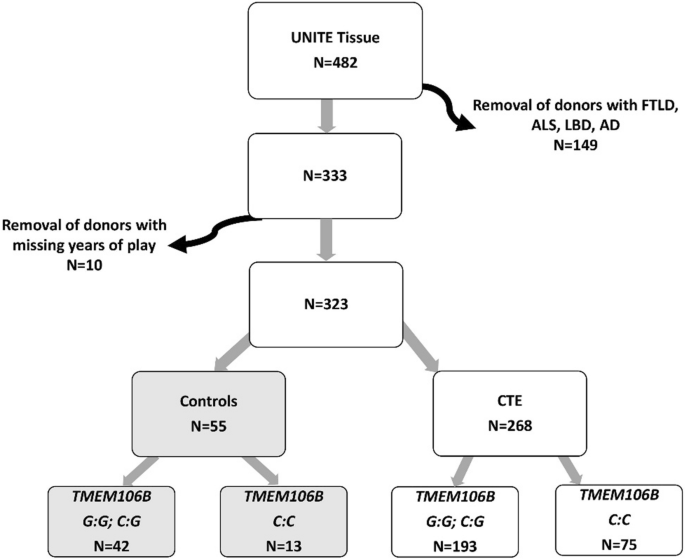
To understand the relationship between genetics and repetitive head impacts (RHI), researchers examined detailed clinical histories and brain tissue from donors in the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Brain Bank.
The study included:
- 323 human brain donors with documented RHI exposure
- 55 donors without CTE pathology
- 268 donors with confirmed CTE pathology
- DNA samples from all donors to determine whether they carried a TMEM106B risk genotype
Researchers also collected extensive retrospective data, including:
- Type of contact sport played
- Level played (high school, college, etc.)
- Number of years at each level
- Clinical symptoms before death
- Cognitive status and dementia history
Alongside genetic analysis, researchers evaluated:
- Microglial activation markers (such as IBA1, CD68, and TREM2)
- Cytokine levels
- Tau pathology, the key hallmark of CTE
- Aβ40 and Aβ42 amyloid proteins
- TDP-43 protein abnormalities, common in several neurodegenerative diseases
The goal was to see whether the TMEM106B variant altered immune responses in the brain—and how those changes connected to actual disease severity.
What TMEM106B Does and Why It Matters
The TMEM106B gene plays a crucial role in the functioning of lysosomes, the cellular structures that act as the brain’s recycling and waste-processing centers. Lysosomes help microglia—the brain’s immune cells—clear debris and maintain healthy neural environments.
When a variation occurs in this gene, the ability of microglia to respond to injury can be compromised. That includes:
- Reduced waste clearance
- Altered inflammatory responses
- Increased risk of abnormal protein buildup
In a brain exposed to repeated trauma, that compromised system can lead to greater damage over time.
Key Findings: How the Variant Affects CTE Severity
The study revealed several important relationships between the TMEM106B risk variant and brain pathology.
1. Older individuals with the risk gene had more severe CTE.
In donors over 65, having the TMEM106B risk genotype:
- More than doubled the likelihood of having more advanced CTE
- Showed a severity effect similar to playing more than eight years of contact sports
- Increased the presence of TDP-43 inclusions, another damaging protein associated with cognitive decline and neurodegenerative disorders
2. Younger individuals with the risk gene were much more likely to have dementia.
For donors 65 or younger, carrying the TMEM106B risk variant:
- Greatly increased the odds of developing dementia, even when overall exposure to head impacts was similar to others
- Suggested that genetics may accelerate the timeline of cognitive decline following repetitive head injuries
This means the gene is not just influencing brain pathology—it’s influencing actual clinical outcomes, including memory loss and cognitive impairment.
TMEM106B and Altered Cytokine Responses
A major contribution of this research is showing exactly how the TMEM106B variant disrupts the brain’s immune system.
The study analyzed cytokine profiles and microglial markers in a subset of 122 donors and found that cytokine–pathology relationships differed dramatically depending on genotype.
In individuals with the protective genotype:
- Cytokines such as IL-6 and IL-8 were positively associated with microglial activation markers
- These cytokines also correlated with tau pathology
- Essentially, inflammation was responding in a more predictable and coordinated way
In individuals with the TMEM106B risk genotype:
- Cytokines including IFN-γ, IL-4, TNF-α, TNF-β, and IL-10 showed negative associations with microglial activation
- TNF-α was negatively associated with tau pathology
- These patterns indicate dysregulated or ineffective inflammatory responses
Such an altered immune environment could make it harder for the brain to clear damaged proteins and recover from trauma, explaining why pathology becomes more severe.
Why This Discovery Is Important
This research provides one of the clearest demonstrations to date that genetics—not just exposure to hits—play a major role in determining who develops severe brain injury outcomes.
Here’s why this matters:
1. It explains differences between individuals with similar exposure.
Two players could have nearly identical sports histories, yet one develops debilitating symptoms while the other does not. This study shows how TMEM106B may be one of the reasons.
2. It provides a potential biomarker for identifying high-risk individuals.
If someone carries the risk variant, they might benefit from:
- Stricter medical monitoring
- More conservative return-to-play decisions
- Early interventions for cognitive symptoms
3. It opens pathways for targeted treatments.
Understanding how microglia misbehave—especially in relation to lysosomal function—could guide:
- Anti-inflammatory therapies
- Drugs that enhance waste clearance
- Precision medicine approaches tailored to genetic risk
4. It deepens our understanding of CTE as a neuroinflammatory disease.
This study reinforces a growing body of work showing that inflammation and immune response are central to CTE progression, not just mechanical injury.
Additional Background: What We Know About TMEM106B Beyond CTE
The TMEM106B gene has been studied in several other neurological conditions, particularly those involving protein misfolding and lysosomal dysfunction.
Research has linked certain TMEM106B variants to:
- Frontotemporal lobar degeneration (FTLD)
- Cognitive decline in aging populations
- Changes in overall brain volume
- Altered microglial activity in disorders involving the progranulin (GRN) gene
This suggests TMEM106B acts as a risk modifier across multiple neurodegenerative pathways, not just CTE. In other words, it may not cause disease on its own, but it can make disease worse when other stressors—such as repeated head injuries—are present.
What This Means Moving Forward
The findings do not suggest that every person with the TMEM106B risk variant will develop CTE or dementia. Likewise, people without the variant are not automatically protected.
But this research does emphasize:
- Genetics matter.
- Inflammation matters.
- Microglia play a central role in brain recovery after trauma.
For athletes, veterans, and anyone exposed to repetitive concussions, this study could mark the beginning of more personalized guidelines for brain health. For scientists, it opens new doors in understanding how the brain’s immune system interacts with long-term trauma.
Research Paper:
Hartman, S., et al. Genetic variation in TMEM106B alters microglial activation and cytokine responses in chronic traumatic encephalopathy. Acta Neuropathologica (2025).
https://doi.org/10.1007/s00401-025-02955-7