How Alcohol Dependence Disrupts Brain Balance by Altering Orexin and Dynorphin Signaling
A growing body of neuroscience research shows that alcohol dependence is not just a matter of willpower but a condition rooted in long-lasting changes in the brain. A new study from The Scripps Research Institute, published in Frontiers in Pharmacology, adds an important piece to this puzzle by showing how alcohol dependence disrupts the delicate balance between two key brain signaling systems. These systems, known as orexin and dynorphin, play opposing roles in motivation, stress, and relapse, and the study reveals how alcohol reshapes their interaction in ways that make staying sober especially difficult.
The researchers focused on understanding why people with alcohol use disorder (AUD) are so vulnerable to relapse, particularly when exposed to stress. Even after long periods without drinking, stress can reignite alcohol-seeking behavior. This study helps explain why that happens by identifying specific molecular and functional changes in the brain that persist well into abstinence.
Alcohol Use Disorder and the Challenge of Relapse
AUD is a chronic condition in which a person continues to drink despite clear physical, psychological, or social harm. In the United States alone, nearly 28 million people are affected. While several FDA-approved medications exist, such as naltrexone, they do not work for everyone and often come with unpleasant side effects like nausea. This has driven scientists to search for new treatment strategies that target the brain mechanisms underlying craving and relapse more precisely.
Stress plays a central role in AUD. On one hand, stress increases the likelihood that someone will start drinking heavily or relapse after quitting. On the other hand, alcohol itself activates the body’s stress systems, creating a vicious cycle. The new study zooms in on how this cycle affects specific neurochemical pathways in the brain.
Orexin and Dynorphin: A Push-Pull System in the Brain
The research centers on two neuropeptides released by the same group of neurons in the hypothalamus, a brain region responsible for coordinating many chemical signals.
- Orexin acts as a motivational “go” signal. It promotes arousal, alertness, and reward-seeking behavior, including drug and alcohol seeking.
- Dynorphin functions more like a “stop” signal. It is associated with aversive feelings, stress, and negative emotional states, especially during withdrawal.
In a healthy brain, these two systems balance each other. Orexin pushes behavior forward, while dynorphin helps rein it in. Alcohol dependence, however, appears to throw this balance off in subtle but powerful ways.
The Brain Region at the Center of the Study: The pPVT
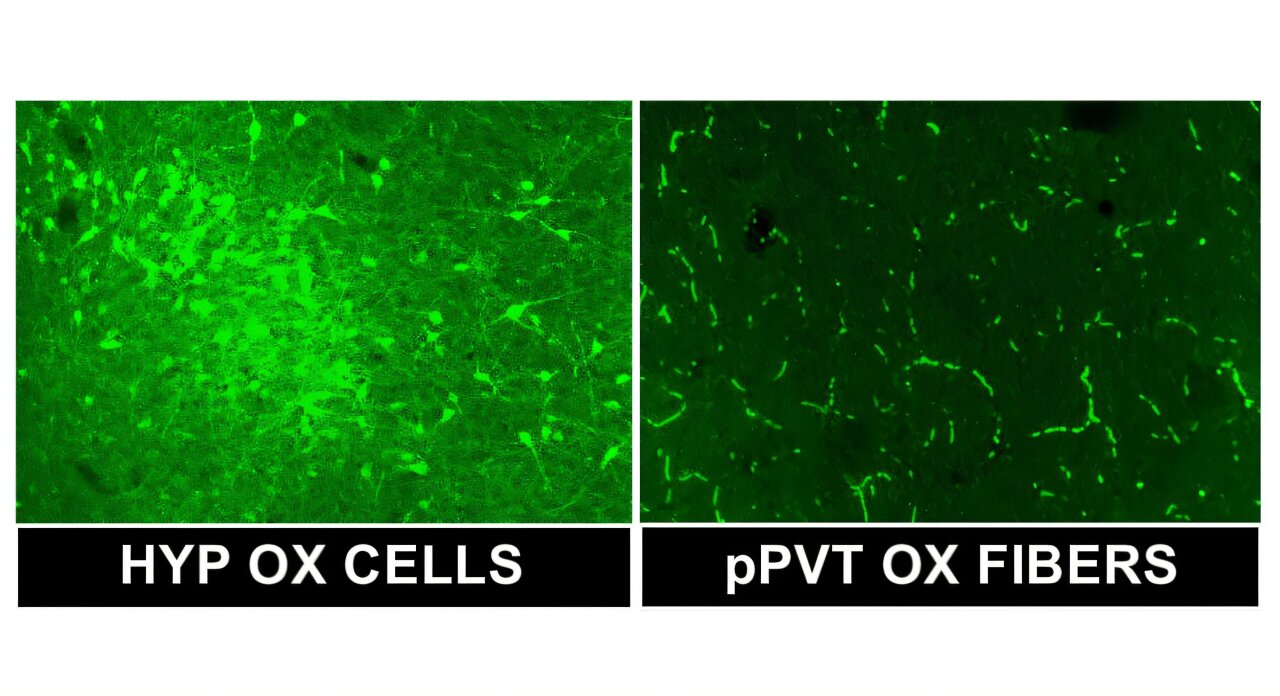
The researchers focused on a small but important brain structure called the posterior paraventricular nucleus of the thalamus, or pPVT. This region is a hub for processing stress and motivation and receives direct input from orexin- and dynorphin-releasing neurons in the hypothalamus.
Previous work from the same research group had already shown that the pPVT plays a critical role in stress-induced relapse-like behavior. In this study, the team wanted to know how alcohol dependence changes signaling in this region at both the molecular and behavioral levels.
How the Study Was Conducted
The experiments were carried out in male rats, which were trained to press a lever to receive alcohol. To model alcohol dependence, the animals were exposed to chronic intermittent alcohol vapor, a well-established method for inducing dependence in laboratory settings. After the alcohol was removed, the rats went through a period of abstinence.
The researchers then examined gene expression in two key brain areas: the hypothalamus, where orexin and dynorphin are produced, and the pPVT, where their signals are received. They also tested how the rats behaved when exposed to stress and whether blocking orexin or dynorphin signaling would change their alcohol-seeking behavior.
Key Molecular Findings
The results revealed striking and long-lasting changes caused by alcohol dependence:
- In the hypothalamus, alcohol-dependent rats showed increased gene expression for both orexin and dynorphin. This suggests that the brain ramps up production of both the “go” and “stop” signals after chronic alcohol exposure.
- In the pPVT, the pattern was different. Neurons expressed fewer receptors for orexin but more receptors for dynorphin.
This mismatch suggests that although more orexin and dynorphin are being produced, the pPVT becomes less sensitive to orexin’s motivational signal and more sensitive to dynorphin’s stress-related effects. Importantly, these changes were observed well into abstinence, indicating that they persist long after drinking stops.
Behavioral Experiments and Surprising Results
To understand how these molecular changes translate into behavior, the researchers directly infused inhibitors into the pPVT to block orexin or dynorphin signaling.
When orexin signaling was blocked, stressed rats showed a clear reduction in relapse-like alcohol seeking, which was expected given orexin’s role in driving motivation. Surprisingly, blocking dynorphin signaling also reduced alcohol-seeking behavior, even though dynorphin is typically thought of as a braking mechanism.
The most unexpected finding came when both systems were blocked at the same time. In this case, the protective effects disappeared entirely. The rats behaved as if they had received no treatment at all, pressing the lever to seek alcohol at the same rate as untreated animals.
This result highlights that orexin and dynorphin do not act independently. Instead, their interaction within the pPVT is complex, and disrupting both simultaneously can cancel out potential benefits.
What This Means for Future Treatments
These findings carry an important warning for drug development. While targeting orexin or dynorphin signaling alone may help reduce relapse risk, combining treatments that affect both systems could have unintended consequences if not carefully designed.
The researchers emphasize that this does not rule out combination therapies altogether. Different drugs, doses, or timing strategies might still prove effective. In fact, ongoing collaborations are exploring short-acting dynorphin pathway inhibitors and how they might work alongside orexin-blocking drugs such as suvorexant, a medication currently approved for insomnia.
Broader Context: Stress, Addiction, and the Brain
This study fits into a larger understanding of addiction as a disorder of stress and reward systems. Chronic alcohol use reshapes brain circuits so that drinking becomes less about pleasure and more about relief from stress and negative emotions. Orexin and dynorphin are central players in this shift, influencing arousal, motivation, and emotional state.
Research on other substances, including cocaine, has shown similar involvement of these systems, suggesting that disrupted orexin-dynorphin balance may be a common feature across different forms of addiction.
Limitations and Next Steps
The researchers note several limitations. The study focused on a single brain region and included only male animals, making it difficult to generalize the findings directly to humans or to females. Nonetheless, the results provide a valuable roadmap for future studies aimed at refining treatments for AUD.
Understanding how specific brain circuits are altered by alcohol dependence brings scientists closer to developing therapies that address the root causes of relapse rather than just the symptoms.
Research paper:
https://www.frontiersin.org/articles/10.3389/fphar.2025.1718540/full