How Anti-Epilepsy Drugs Physically Reshape a Crucial Brain Protein

Researchers have long known that several widely used anti-epilepsy drugs work by targeting a protein called SV2A, but exactly how those drugs affect the protein at a structural level remained a mystery. A new study has now filled in that gap. Using advanced imaging techniques, scientists have revealed how these medications physically reshape SV2A when they bind to it—and how this knowledge could guide the development of more precise and effective treatments for epilepsy in the future.
The research, published in Nature Communications, was carried out by a multi-institute team led by St. Jude Children’s Research Hospital in collaboration with UT Southwestern Medical Center. The team used cryo-electron microscopy (cryo-EM) to visualize SV2A in unprecedented detail, both on its own and when bound to several anti-seizure drugs and experimental modulators.
Why SV2A Matters in Epilepsy Treatment
SV2A, short for synaptic vesicle glycoprotein 2A, is a membrane protein found in nearly all neurons. It is especially abundant on synaptic vesicles—the tiny structures that store and release neurotransmitters at synapses. Because seizures arise from abnormal electrical signaling in the brain, proteins involved in neurotransmitter release are critical points of intervention.
Several anti-epilepsy drugs, including levetiracetam and brivaracetam, are known to bind to SV2A. These drugs are widely prescribed because they can reduce seizure frequency without many of the side effects associated with older therapies that act on sodium channels or GABA receptors. Despite their clinical importance, SV2A’s biological role is still not fully understood, and its natural substrate has not yet been identified.
This gap in understanding made SV2A a particularly intriguing target for structural studies.
Using Cryo-EM to See Drug-Protein Interactions
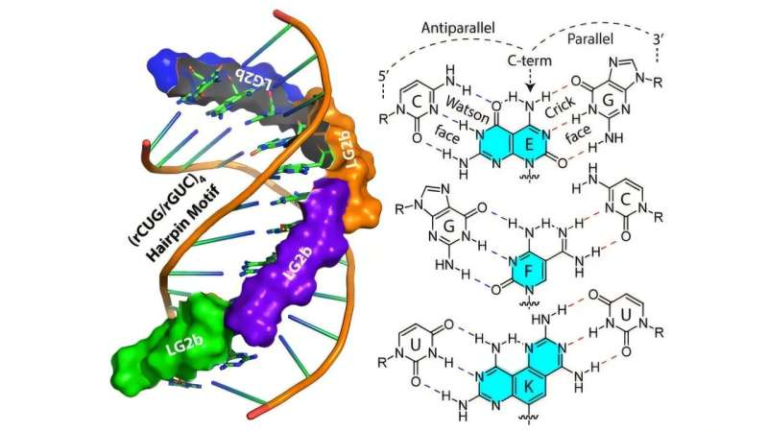
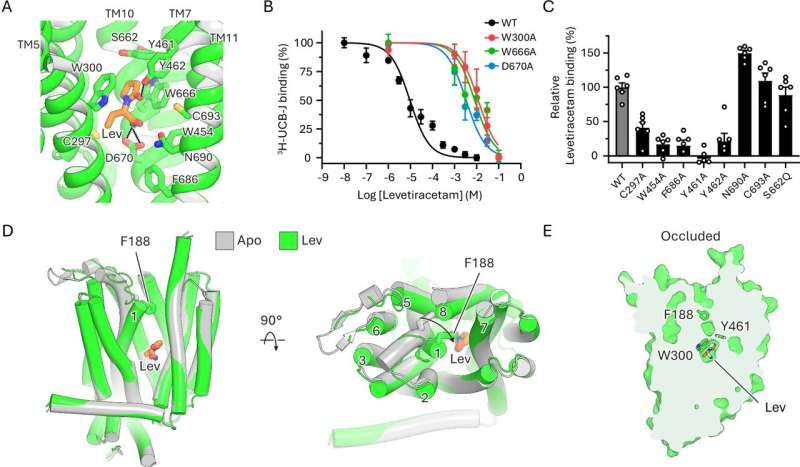
To uncover what happens when anti-seizure drugs bind to SV2A, the researchers turned to cryo-electron microscopy, a technique that allows scientists to visualize proteins at near-atomic resolution by flash-freezing them and imaging them with electron beams.
The team solved multiple structures of human SV2A:
- SV2A on its own
- SV2A bound to FDA-approved anti-seizure drugs
- SV2A bound to experimental modulators designed to enhance drug activity
These structures revealed, for the first time, the exact conformational changes that occur in SV2A when drugs attach to it.
Primary and Allosteric Binding Sites Explained
One of the most important findings of the study is that SV2A contains two distinct functional binding regions.
The first is the primary binding site, where well-known anti-epilepsy drugs such as levetiracetam and brivaracetam attach. When these drugs bind, SV2A undergoes structural rearrangements typical of proteins in the major facilitator superfamily, a large group of transporter-like proteins found across biology.
The second is an allosteric site, a separate region of the protein that does not directly compete with the main drug-binding site. Instead, molecules binding here can subtly change the protein’s shape in ways that influence how effectively drugs bound at the primary site work.
This secondary site turned out to be key for understanding why some drug combinations are more potent than others.
Why Some Drugs Behave Differently
By comparing how different compounds interact with SV2A, the researchers uncovered important distinctions:
- Levetiracetam and brivaracetam bind exclusively to the primary site and trigger the expected transporter-like structural changes.
- Certain experimental modulators bind only to the allosteric site and enhance the effectiveness of levetiracetam and brivaracetam when used together.
- The investigational anti-seizure drug padsevonil behaves differently. It binds to both the primary and allosteric sites, resulting in a unique structural configuration.
Interestingly, the allosteric modulators that improved levetiracetam and brivaracetam did not enhance padsevonil’s effects. This finding highlights the complexity of SV2A–drug interactions and explains why not all SV2A-targeting drugs respond the same way to additional modulators.
What This Means for Drug Design
The study provides practical guidance for developing next-generation epilepsy treatments. One key insight is that the primary binding site is highly conserved across related transporter proteins, while the allosteric site shows more variation.
This has major implications:
- Drugs targeting the primary site may also affect other proteins in the same family, increasing the risk of side effects.
- Drugs designed specifically for the allosteric site could be more selective, reducing unintended interactions and improving safety.
By focusing on the allosteric site, researchers may be able to design therapies that are more specific to SV2A, offering better seizure control with fewer off-target effects.
Levetiracetam’s Unique Place in Medicine
The findings are particularly relevant given the importance of levetiracetam in modern medicine. It is:
- One of the most commonly prescribed anti-epilepsy drugs worldwide
- Included on the World Health Organization’s Essential Medicines List
- The first and only FDA-approved 3D-printed drug, a milestone in pharmaceutical manufacturing
Understanding exactly how levetiracetam alters SV2A at a structural level adds an extra layer of confidence to its continued use and opens doors for improving upon it.
Still a Mystery: What Does SV2A Actually Do?
Despite decades of clinical use of SV2A-binding drugs, the protein’s native biological function remains unclear. It is thought to act as a transporter or regulator of synaptic vesicle function, but definitive proof is still lacking.
The researchers emphasize that better inhibitors and modulators are not just useful as therapies—they are also tools for basic science. By selectively turning SV2A activity up or down, scientists can better investigate its role in neuronal signaling and synaptic health.
Why This Research Matters Beyond Epilepsy
While the study focuses on epilepsy, its implications extend further. SV2A is expressed throughout the brain, and abnormal synaptic transmission is involved in many neurological conditions. Insights into SV2A structure and modulation could eventually inform research into neurodevelopmental disorders, neurodegenerative diseases, and other conditions where synaptic dysfunction plays a role.
At a broader level, the study demonstrates how structural biology can bridge the gap between molecular details and real-world medical outcomes.
Looking Ahead
The research team plans to continue exploring SV2A to determine whether it truly functions as a transporter and how its activity is regulated inside neurons. Each new structural insight brings scientists closer to understanding a protein that has quietly played a central role in epilepsy treatment for years.
As our picture of SV2A becomes clearer, so does the path toward more precise, more effective, and more personalized anti-seizure therapies.
Research Paper:
https://www.nature.com/articles/s41467-025-65781-1