How Beige Fat Helps the Body Keep Blood Pressure Under Control
Obesity and high blood pressure are closely linked, and together they drive a large share of cardiovascular disease, the leading cause of death worldwide. While doctors and researchers have long recognized this connection, the precise biological reason why excess fat raises blood pressure has remained surprisingly unclear. A new study from The Rockefeller University, published in Science, now offers a detailed and compelling explanation. The key player is not just fat in general, but a specific kind of fat known as beige fat, which appears to play a direct role in protecting blood vessels and regulating blood pressure.
The research shows that when beige fat is lost, blood vessels become overly sensitive to powerful hormones that constrict them, leading to stiffer arteries and higher blood pressure. Importantly, this happens independently of obesity, suggesting that the type of fat surrounding blood vessels may matter as much as, or more than, the total amount of fat in the body.
Why Beige Fat Is Different From Other Fat
Not all fat tissue behaves the same way. Most people are familiar with white fat, which stores excess calories and expands in obesity. In contrast, brown fat burns energy to produce heat and is abundant in newborns and animals exposed to cold. Adults also retain some thermogenic fat, and in humans this is often referred to as inducible brown fat.
In mice, the closest equivalent to adult human brown fat is beige fat. Beige fat cells can switch between energy-storing and energy-burning modes, and they are commonly found in perivascular adipose tissue (PVAT), the fat that wraps around blood vessels.
Previous clinical studies had shown that people with more brown fat tend to have lower odds of hypertension and other cardiometabolic diseases, but these studies could only demonstrate correlation. What they could not answer was whether thermogenic fat actively controls blood pressure, or whether it is simply a marker of better overall health.
A Mouse Model Designed to Isolate Beige Fat
To move from correlation to causation, researchers led by Paul Cohen and Mascha Koenen engineered a precise mouse model. Instead of comparing obese and lean animals, they created mice that were metabolically healthy in every way except one: their fat cells could no longer maintain a beige identity.
This was achieved by deleting PRDM16, a gene that is essential for beige fat identity, specifically in adipocytes. As a result, beige fat cells lost their thermogenic characteristics and began to resemble white fat cells instead. This design allowed the researchers to isolate the effect of beige fat itself, without confounding factors such as inflammation, insulin resistance, or excess weight.
The consequences of this seemingly subtle change were striking.
Loss of Beige Fat Triggers High Blood Pressure
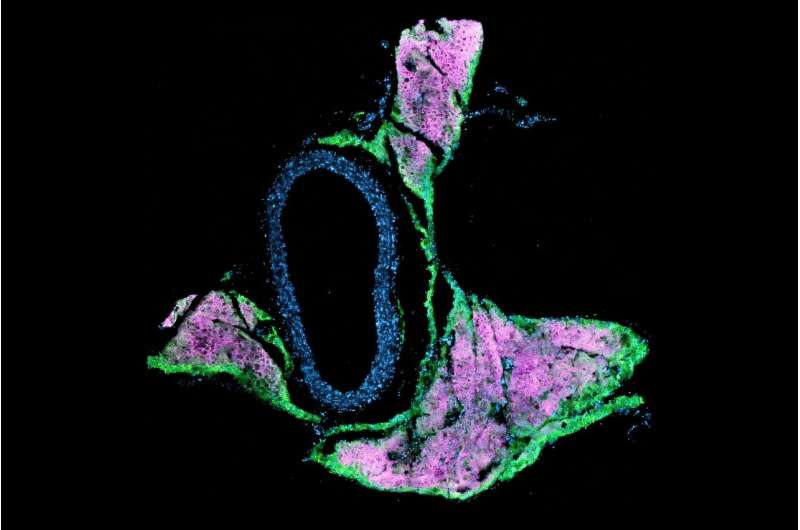
Mice lacking beige fat identity developed elevated blood pressure and increased mean arterial pressure. When researchers examined the blood vessels more closely, they found clear signs of vascular remodeling, including the buildup of stiff, fibrous tissue around the arteries.
Even more telling was how the blood vessels responded to angiotensin II, one of the body’s most potent vasoconstricting hormones and a central player in blood pressure regulation. Arteries from these mice showed hypersensitivity to angiotensin II, constricting far more strongly than normal vessels. This exaggerated response makes blood flow more difficult and forces the heart to work harder, driving blood pressure upward.
Single-nucleus RNA sequencing revealed that, in the absence of beige fat, vascular cells switched on gene programs associated with fibrosis and stiffness, further confirming that the vessels themselves had fundamentally changed.
Identifying the Molecular Culprit: QSOX1
The next challenge was to identify what signal from fat cells was driving these vascular changes. The researchers focused on factors secreted by adipocytes that had lost their beige identity. By exposing vascular cells to fluid released from these altered fat cells, they found that this fluid alone was sufficient to activate fibrosis-related genes.
Through large-scale gene and protein expression analyses, the team pinpointed a single enzyme: QSOX1. This enzyme, previously linked to tissue remodeling in cancer, was found to be tightly regulated by beige fat. Under normal conditions, beige fat keeps QSOX1 expression low. When beige identity is lost, QSOX1 levels rise sharply.
To confirm that QSOX1 was truly responsible, the researchers engineered mice that lacked both PRDM16 and QSOX1. These mice still lacked beige fat, but crucially, they did not develop vascular dysfunction or high blood pressure. This result demonstrated that QSOX1 is a key mediator linking beige fat loss to hypertension.
Evidence That the Findings Apply to Humans
Although the experiments were conducted in mice, the study also included human data. Analysis of large clinical cohorts showed that people carrying mutations in PRDM16 tend to have higher blood pressure. This strengthens the case that the beige fat–QSOX1 pathway identified in mice is relevant to human physiology as well.
These findings help explain why earlier imaging studies consistently found lower rates of hypertension in people with detectable brown fat. Beige and brown fat appear to actively protect blood vessels, not merely coexist with good cardiovascular health.
Why Perivascular Fat Matters So Much
The fat surrounding blood vessels is increasingly recognized as an active organ rather than passive insulation. PVAT communicates directly with the vessel wall, influencing vascular tone, inflammation, and structural integrity. When PVAT is healthy and beige-like, it helps maintain flexible, responsive arteries. When it shifts toward a white-fat state, it can promote fibrosis, stiffness, and disease.
This study adds a crucial piece to that puzzle by showing how changes in PVAT identity can directly alter hormone sensitivity and vessel behavior.
Broader Implications for Treating Hypertension
Hypertension is currently treated largely by targeting hormones and receptors within the cardiovascular system itself, such as angiotensin receptors or calcium channels. The new findings suggest an additional and potentially powerful strategy: targeting fat–vessel communication.
In particular, QSOX1 emerges as a promising therapeutic target. Inhibiting this enzyme could theoretically prevent or reverse vascular stiffening without needing to broadly alter blood pressure signaling throughout the body. Another long-term possibility is developing approaches that preserve or enhance beige fat identity around blood vessels.
While these ideas are still at an early stage, the study provides a clear molecular framework for future research and drug development.
What This Study Changes in Our Understanding
For decades, obesity-related hypertension was viewed mainly as a consequence of excess weight, inflammation, and metabolic dysfunction. This research challenges that view by showing that the loss of a protective fat type alone can raise blood pressure, even in the absence of obesity.
It reframes fat not just as a risk factor, but as an active regulator of vascular health. Understanding these distinctions could eventually lead to more precise, personalized treatments for cardiovascular disease.
Research Reference
Ablation of Prdm16 and beige fat identity causes vascular remodeling and elevated blood pressure
https://www.science.org/doi/10.1126/science.ady8644