How Genetics and AI Are Transforming the Diagnosis of Aortic Stenosis

Aortic stenosis is one of the most common and serious forms of heart valve disease, affecting millions of people worldwide. It occurs when the aortic valve, which regulates blood flow from the heart to the rest of the body, becomes narrowed. Over time, this narrowing forces the heart to work harder, eventually leading to heart failure or death if left untreated. Despite its severity, modern medicine still lacks effective drugs to prevent or slow the disease. Treatment usually begins only once the valve is severely damaged, typically through surgery or catheter-based valve replacement.
A new study led by researchers from the University of California, San Francisco (UCSF) and the Broad Institute of MIT and Harvard offers a promising shift in how aortic stenosis may be understood, diagnosed, and potentially prevented. By combining genetic research with artificial intelligence, the team uncovered a detailed genetic foundation that links normal variations in aortic valve function to the eventual development of aortic stenosis.
Understanding the Challenge of Studying Aortic Stenosis
One of the biggest challenges in researching aortic stenosis is that severe disease is relatively rare at the population level. This makes it difficult to identify genetic risk factors using traditional approaches that rely only on diagnosed cases. To overcome this limitation, the researchers took a different approach.
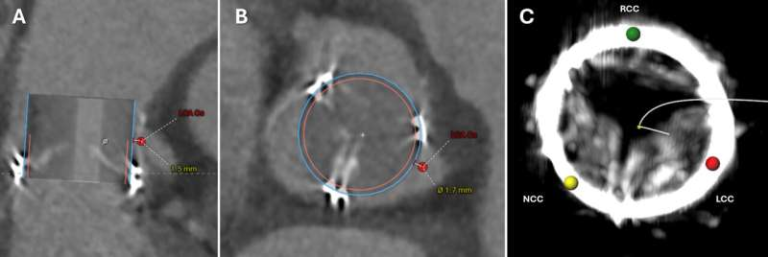
Credit: Nature (2025)
Instead of focusing solely on people who already had the disease, they studied normal variations in aortic valve function among healthy individuals. The idea was simple but powerful: if certain genetic variants influence how well the valve works in healthy people, those same variants might also contribute to disease risk later in life.
Using AI to Measure Valve Function at Scale
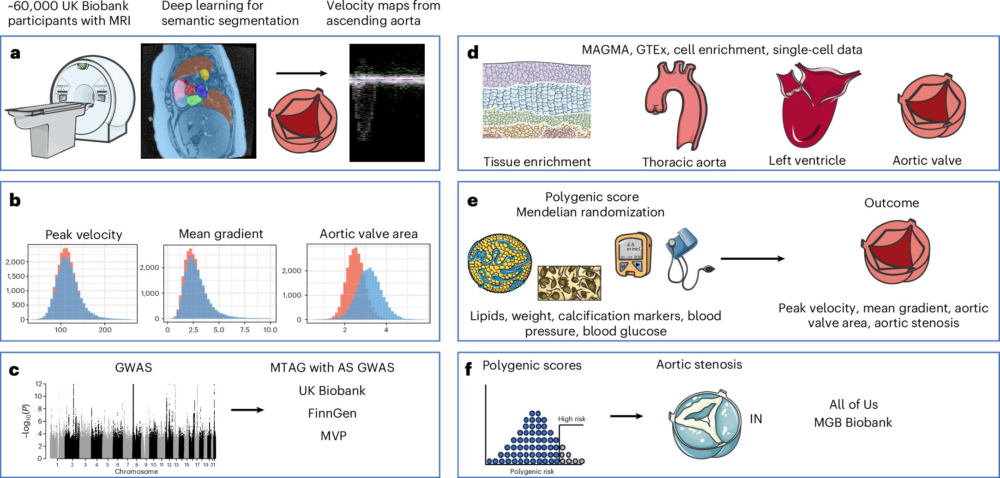
To make this possible, the researchers relied heavily on deep learning, a form of artificial intelligence particularly suited for analyzing medical images. They applied AI models to cardiac MRI scans from nearly 60,000 healthy participants in the UK Biobank, one of the world’s largest biomedical databases.
The AI models extracted three key measurements of aortic valve function:
- Peak velocity, which reflects how fast blood flows through the valve
- Mean gradient, a measure of pressure difference across the valve
- Aortic valve area (AVA), which indicates how open or narrow the valve is
These measurements are commonly used in clinical settings, but manually extracting them at this scale would have been nearly impossible. AI made it feasible to generate consistent, high-quality measurements across tens of thousands of people.
Large-Scale Genetic Analysis and Major Discoveries
Once these measurements were available, the team conducted genome-wide association studies (GWAS) to identify genetic variants linked to valve function. This analysis revealed 61 distinct genetic loci associated with the three AI-derived valve traits.
To connect these findings directly to disease, the researchers also performed a massive meta-analysis of aortic stenosis diagnoses, involving more than 40,000 cases and 1.5 million controls drawn from multiple biobanks around the world. This disease-focused analysis identified 91 genetic loci associated with aortic stenosis.
The most powerful results came from a multi-trait analysis that combined both approaches—healthy valve measurements and disease diagnoses. This integrated analysis uncovered 166 genetic loci in total, with 134 linked to aortic valve function and 134 linked to aortic stenosis, many of them overlapping. This overlap strongly suggests that the genetics shaping normal valve variation are deeply connected to the genetics driving disease.
Strong Genetic Links Between Normal Function and Disease
One of the clearest findings from the study was the high genetic correlation between valve function in healthy individuals and diagnosed aortic stenosis. On a scale from 0 to 1, where higher numbers indicate stronger shared genetic influence:
- Gradient-based measurements showed a correlation of 0.64 with aortic stenosis
- Aortic valve area showed a correlation of 0.50
These are substantial correlations, reinforcing the idea that aortic stenosis does not suddenly appear but rather develops along a continuum shaped by genetics long before symptoms arise.
Connections to Cholesterol, Heart Disease, and Phosphate Biology
Beyond identifying genetic loci, the study also revealed meaningful biological connections. Many of the genetic signals associated with aortic stenosis overlapped with pathways involved in:
- Coronary artery disease
- Lipoprotein and cholesterol biology, including well-known cardiovascular genes
- Phosphate handling and metabolism, which plays a role in tissue calcification
These findings are especially intriguing because they point to potentially modifiable risk factors. While no medical therapies currently exist to prevent aortic stenosis, these pathways could guide future research into treatments aimed at slowing or preventing valve narrowing before it becomes severe.
Importantly, the researchers emphasized that clinical validation is still required. Any future strategies involving cholesterol or phosphate manipulation would need rigorous testing before being applied in patient care.
Why This Study Matters for the Future of Diagnosis
Traditionally, aortic stenosis is diagnosed only after the valve has become significantly narrowed, often when symptoms like chest pain, shortness of breath, or fainting appear. By then, intervention is usually unavoidable.
This study highlights a future where genetics and AI-driven imaging could help identify people at higher risk much earlier—possibly decades before severe disease develops. It also demonstrates the power of combining cardiovascular structure, function, and genetic data into a single analytical framework.
Extra Insight: Why Aortic Stenosis Is So Difficult to Treat
Aortic stenosis is particularly challenging because it involves progressive calcification of the valve. Unlike clogged arteries, which can sometimes be managed with medications and lifestyle changes, calcified valves do not respond well to current drug therapies. Once the valve becomes stiff and narrow, mechanical intervention is the only effective solution.
This makes early detection especially important. Understanding who is genetically predisposed could eventually lead to preventive strategies, even if those strategies are still years away.
Extra Insight: The Growing Role of AI in Cardiology
This research also reflects a broader trend in medicine. Artificial intelligence is rapidly becoming a key tool in cardiology, especially in imaging-heavy fields like echocardiography and cardiac MRI. AI can detect subtle patterns invisible to the human eye, standardize measurements across populations, and dramatically increase research scale.
In this case, AI didn’t just speed up analysis—it enabled discoveries that would have been impossible using traditional methods alone.
Looking Ahead
While this research does not immediately change how aortic stenosis is treated, it significantly advances understanding of how the disease develops at a genetic level. It also provides a strong example of how AI and genetics together can uncover hidden connections between normal biology and disease.
As large biobanks grow and AI tools continue to improve, studies like this may redefine how cardiovascular diseases are predicted, studied, and eventually prevented.
Research paper:
https://www.nature.com/articles/s41588-025-02397-7