How Parents Pass Longevity to Their Offspring: New Findings in Roundworm Research
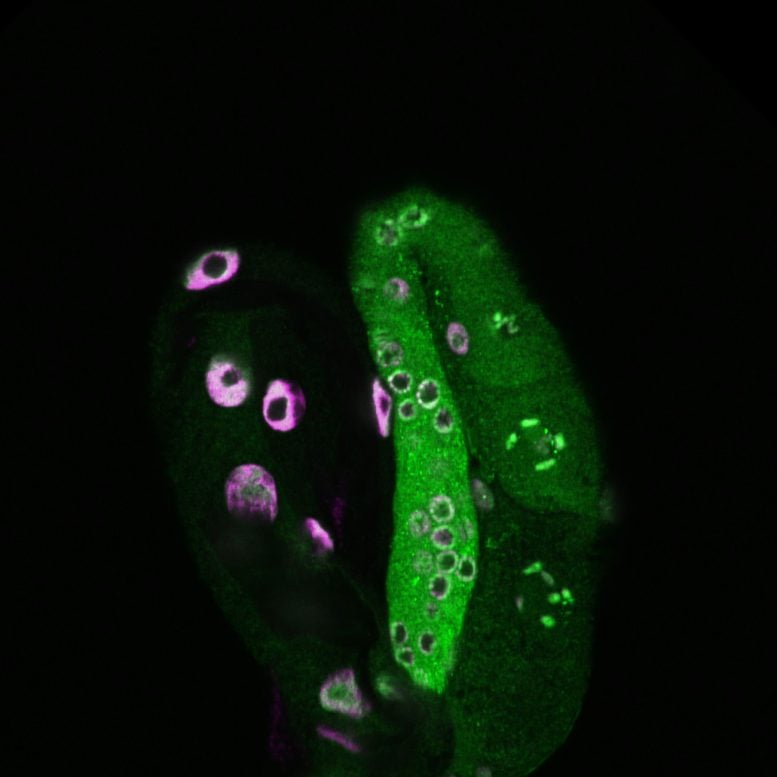
A fascinating new study has revealed that parents can pass on traits related to longer lifespans to their offspring—not through DNA sequence changes, but via epigenetic mechanisms tied to the activity of lysosomes. Conducted in the tiny roundworm Caenorhabditis elegans (C. elegans), this research sheds light on how the body’s physiological state can influence future generations. What makes it especially exciting is that it provides a molecular explanation for how environmental experiences such as fasting or stress might be encoded and inherited, without changing the genetic code itself.
This discovery, led by Meng Wang and colleagues at the Howard Hughes Medical Institute’s Janelia Research Campus, has major implications for how we understand inheritance, aging, and even the long-term effects of diet and stress on descendants. Let’s take a closer look at what they found, how they did it, and why it matters.
The Core Discovery
The study, published in Science in September 2025, describes a mechanism where changes in lysosomes—the cellular compartments usually known for recycling waste—can influence lifespan and transmit that advantage across generations.
The researchers worked with worms engineered to overexpress an enzyme in lysosomes. This tweak extended the lifespan of the worms by nearly 60 percent. Interestingly, even when these genetically altered worms were crossed with normal worms, their offspring also lived longer, despite not carrying the genetic modification. The effect persisted for up to four generations.
This indicated that something other than DNA sequence was being transmitted—a form of epigenetic inheritance. The team discovered that histones (proteins that package DNA) play a key role here. Specifically, lysosomal activity influenced a histone variant, H3.3, and a chemical mark on histones called H3K79 methylation. These changes were then carried into reproductive cells, allowing the information to be passed on.
How It Works: From Lysosome to Germline
Here’s the step-by-step breakdown of what happens inside the worm:
- Lysosomal metabolism changes – Alterations in lysosome function (like boosting certain enzymes, fasting, or suppressing nutrient-sensing pathways) trigger a chain of intracellular events.
- Histone H3.3 is upregulated – This histone variant is produced in greater amounts.
- Transport to germline cells – H3.3 is moved from the worm’s somatic tissues (such as the intestine) into the germline (cells that will become sperm or eggs).
- H3K79 methylation is added – Once inside germline cells, a specialized methyltransferase enzyme modifies H3.3 at the lysine 79 position.
- Epigenetic inheritance – These modified histones embed the lysosomal “memory” into reproductive cells, ensuring that the next generation inherits the longevity-associated traits.
The researchers also showed that if they directly increased H3.3 levels or overexpressed the methyltransferase enzyme, the worms lived longer—even without lysosomal modifications. This confirmed that the histone changes were the crucial link.
The Role of Fasting and Environmental Stress
The study also tied this pathway to fasting. When worms experienced food deprivation, their lysosomes shifted into a mode that promoted the same histone modifications. The result? Their offspring inherited the lifespan-extending benefits.
This shows how environmental experiences—like changes in diet—can leave a molecular mark on future generations. It provides a clear mechanistic link between external stressors and transgenerational epigenetic inheritance.
Why Lysosomes Matter More Than We Thought
For decades, lysosomes were seen mainly as the cell’s “trash disposal” units. They break down old proteins, recycle cellular debris, and keep cells clean. But over the past 15 years, scientists have realized that lysosomes are much more than garbage processors—they are also signaling hubs.
This study reinforces that idea. Lysosomes appear to act as sensors of nutrient status and stress, relaying information to the nucleus and influencing gene expression. Now, we know they can also affect epigenetic programming in germline cells, effectively linking the soma (body) with the germline (reproductive system).
Broader Implications
The implications of this discovery stretch beyond worms:
- Aging research: Understanding how histone modifications extend lifespan in worms could point toward strategies for improving longevity in more complex organisms.
- Health and disease: If similar processes occur in humans, parental experiences with diet, stress, or toxins might influence their children’s health in ways we don’t yet fully appreciate.
- Epigenetic inheritance: This provides one of the clearest molecular explanations for how traits can be passed across generations without DNA changes.
Of course, there’s a big caveat: humans are not worms. While C. elegans is a powerful model organism, translating these results to mammals will take years of additional research. Still, the basic principles of lysosomal signaling and histone modification are highly conserved across species.
Extra Knowledge: What Are Lysosomes?
To fully grasp the importance of this finding, it helps to understand what lysosomes are.
- Lysosomes are membrane-bound organelles found in nearly all animal cells.
- They contain digestive enzymes that break down biomolecules like proteins, lipids, and carbohydrates.
- Lysosomes recycle materials, providing building blocks for new cellular structures.
- They play a role in cellular processes like autophagy, where the cell digests its own damaged parts.
- Recent studies show lysosomes are metabolic sensors, interacting with nutrient-sensing pathways such as mTOR and AMPK, which regulate growth, metabolism, and aging.
This makes them critical not just for cellular “housekeeping” but also for decisions about growth, repair, and survival.
Extra Knowledge: What Is Epigenetic Inheritance?
Another important concept here is epigenetics. Unlike genetic inheritance (which is DNA-based), epigenetic inheritance involves chemical tags and modifications that regulate how genes are turned on or off.
Key forms of epigenetic regulation include:
- Histone modifications (like acetylation, methylation, phosphorylation).
- DNA methylation (adding methyl groups to DNA bases, usually silencing genes).
- Non-coding RNAs that regulate gene expression.
Epigenetic changes don’t alter the DNA sequence itself. Instead, they affect gene activity. And under certain circumstances, these modifications can be passed from parent to offspring, influencing traits across generations.
The C. elegans study is a perfect example of this, showing how histone changes caused by lysosomal activity can be inherited.
Extra Knowledge: Why Use Worms in Aging Research?
You might wonder why researchers often turn to C. elegans for studies on longevity and inheritance. Here’s why:
- They have a short lifespan (2–3 weeks), which makes it easy to study aging and multiple generations in a short time.
- Their genome is fully mapped, and they share many fundamental biological pathways with humans.
- They are transparent, allowing researchers to track changes in cells under a microscope.
- Their biology is relatively simple, but the mechanisms they use for gene regulation, epigenetics, and metabolism are highly conserved.
This makes them an ideal system to uncover fundamental biological principles, which can later be tested in more complex animals.
Limitations and Open Questions
While this study provides strong evidence for a histone-based mechanism of transgenerational inheritance, many questions remain:
- How long do the effects last? The study showed up to four generations, but does it fade beyond that?
- How universal is the mechanism? Do other organisms use similar lysosome-to-histone pathways?
- What about reversibility? Can these inherited changes be undone if the environment shifts dramatically?
- Human relevance: While conserved pathways suggest potential parallels, direct evidence in mammals is still lacking.
These are important areas for future research.
Why This Matters for Everyday Life
Although we’re still in the realm of worm biology, the implications for humans are hard to ignore. If similar pathways exist in us, it could mean that our lifestyle choices—diet, stress levels, exposure to toxins—don’t just affect us, but also our children and grandchildren.
It reframes the old idea of inheritance. Instead of being limited to what’s written in DNA, it suggests that our life experiences can leave molecular footprints that echo across generations.
Reference
Research paper: Lysosomes signal through the epigenome to regulate longevity across generations