How Physical Pressure Reprograms Cancer Cells Into More Invasive States

Cancer research has taken another fascinating turn. A new study published in Nature reveals that cancer cells don’t just change because of chemical signals or genetic mutations. They can also shift behavior when exposed to mechanical pressure—the physical squeeze from surrounding tissues. This pressure forces them into a new, more dangerous state: less focused on rapid growth but more prone to invasion and drug resistance.
This discovery adds a whole new dimension to how we think about cancer biology. For years, scientists believed that epigenetic changes—alterations in how genes are expressed without modifying the underlying DNA sequence—were mainly driven by chemical processes inside cells. Now, it’s clear that physical confinement itself can flip genetic switches.
The Research at a Glance
The study, titled Mechanical confinement governs phenotypic plasticity in melanoma, was led by Richard White of Ludwig Oxford and Miranda Hunter of Memorial Sloan Kettering Cancer Center, along with collaborators across multiple institutions.
They worked with both zebrafish models of melanoma and human melanoma cells in controlled lab experiments. By focusing on cells under tight confinement, the researchers uncovered how tumors physically pressed by nearby tissues adapt in unexpected ways.

Instead of continuing their usual pattern of uncontrolled division, these cells activated a program resembling neuronal invasion. That means they reorganized themselves to move more effectively through tissues, resembling how nerve cells migrate during development.
The central player in this transformation turned out to be HMGB2, a protein that bends DNA and changes how tightly it is packaged in the nucleus. Elevated HMGB2 levels unlocked previously hidden regions of the genome, exposing invasive behaviors at the expense of growth.
How They Tested the Idea
To confirm these observations, the team used a variety of approaches:
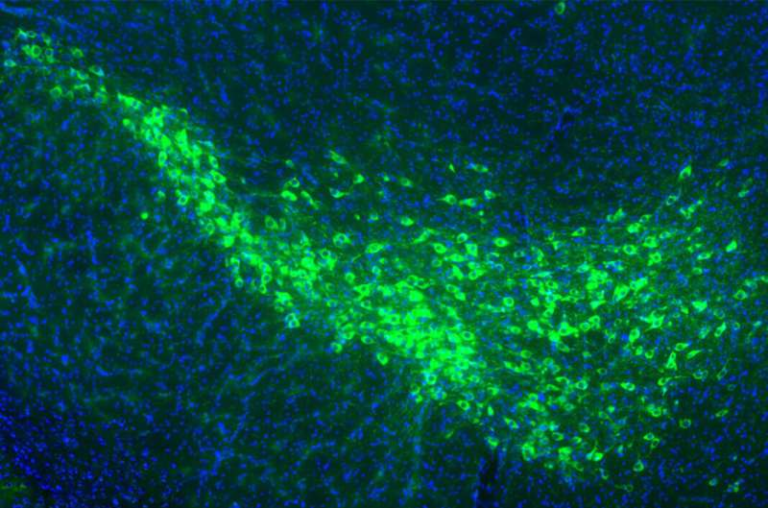
- Zebrafish tumor models: The researchers identified special “interface cells” sitting at the tumor’s edge, pressed tightly by surrounding tissue. These cells displayed elongated nuclei—clear signs of mechanical stress. Spatial transcriptomics revealed these interface cells were genetically different from the freely growing ones deeper inside the tumor.
- Lab confinement tests: Human melanoma cells (A375) were squeezed under a polydimethylsiloxane piston at a height of just 3 microns. Despite the squeeze, the cells did not die off. Instead, they dramatically changed their internal structure and gene expression.
- Gene expression analysis: Confined cells activated a set of genes usually associated with neuronal development and invasion, including SOX11, NNAT, NEUROD1, and NEFM. At the same time, pathways linked to rapid proliferation were dialed down.
- Microscopy: Under mechanical stress, melanoma cells reorganized their cytoskeleton. Microtubules formed a cage-like structure around the nucleus, particularly rich in acetylated tubulin. This adaptation seemed to protect the nucleus from rupture while helping the cell squeeze through tight spaces.
- Drug resistance testing: The confined melanoma cells showed greater resistance to Taxol (paclitaxel), a chemotherapy drug that targets dividing cells by stabilizing microtubules. In animal models, cells with higher HMGB2 also resisted targeted melanoma treatments like dabrafenib and trametinib.
The Role of HMGB2
One of the most striking discoveries was the role of HMGB2. This protein normally helps bend DNA and regulate how genetic material is packaged. Under confinement, HMGB2 levels rose significantly.
Confined cells with high HMGB2 levels became less proliferative but more invasive. In other words, they shifted strategy: instead of dividing quickly, they focused on spreading. This shift also made them tougher against certain cancer drugs, which usually target fast-dividing cells.
Knocking down HMGB2 altered this balance. Cells with reduced HMGB2 didn’t undergo the same invasive transformation, showing that this protein is a key mechanosensitive regulator of cancer plasticity.
Remodeling the Skeleton of the Cell
The cytoskeleton is the network of fibers that gives cells their shape and allows them to move. Under confinement, melanoma cells remodeled their microtubules into a stabilized, protective cage around the nucleus.
This response depended on the enzyme ATAT1, which acetylates tubulin to make microtubules more stable. Without ATAT1, cells struggled to maintain this perinuclear shield.
Interestingly, this adaptation closely resembled neuronal architecture, where microtubules also form non-centrosomal, protective structures. This similarity reinforces the idea that confined cancer cells tap into ancient, developmentally conserved programs to survive and spread.
Why This Matters for Cancer Treatment
Traditional cancer therapies often target cells that divide rapidly. That makes sense because unchecked proliferation is one of cancer’s hallmarks. But this study highlights a problem: under pressure, cancer cells can switch states.
- Fast-dividing cells are vulnerable to chemotherapy.
- Confinement-stressed cells stop dividing as much and instead become invasive and resistant.
That means some treatments could wipe out the obvious fast growers but leave behind a dangerous population of invasive, therapy-resistant cells. These survivors could drive relapse and metastasis.
The research suggests that to outsmart cancer, therapies will need to also target the mechanical and epigenetic pathways—such as HMGB2 and microtubule remodeling—that help cells escape traditional treatments.
Funding and Support
This ambitious study drew support from a wide range of organizations, including:
- Ludwig Institute for Cancer Research
- National Cancer Institute
- Cancer Research Society
- Canadian Institutes of Health Research
- U.S. National Institutes of Health
- Melanoma Research Alliance
- Debra and Leon Black Family Foundation
- Pershing Square Sohn Foundation
- The Mark Foundation
- The Alan and Sandra Gerry Metastasis Research Initiative
- The Harry J. Lloyd Foundation
- Consano
- Starr Cancer Consortium
- American Cancer Society
This level of collaboration highlights just how seriously the scientific community is taking the role of the tumor microenvironment in cancer progression.
Bigger Picture: Cancer Plasticity
The phenomenon observed here is part of a larger issue known as cancer plasticity. Cancer cells are remarkably flexible. They can shift between different states depending on cues from their surroundings.
Plasticity makes them difficult to treat. Even if you design a drug to target one state (fast-dividing cells, for example), other cells can switch into an alternative state (slow-growing, invasive, or drug-resistant) and survive.
The new study shows that mechanical confinement is a powerful cue driving this kind of switching. This wasn’t fully appreciated before. Most attention has gone to chemical signals, oxygen levels, or immune interactions. Now physical pressure itself joins the list of critical drivers.
Beyond Cancer: Mechanical Stress in Biology
While this study focuses on melanoma, the concept of mechanical stress influencing biology is broader. In fact, many normal biological processes rely on mechanical cues:
- Embryonic development: Cells migrate and change shape as tissues fold and grow. Mechanical forces guide this choreography.
- Wound healing: Fibroblasts sense stiffness in scar tissue and adjust their behavior accordingly.
- Stem cell fate: Stem cells on stiff surfaces often turn into bone cells, while those on soft surfaces may become fat cells.
So it’s not surprising that cancer cells—masters of adaptation—also harness mechanical signals to their advantage. The twist is that they use these signals to become more invasive and more dangerous.
What’s Next?
The findings raise several open questions:
- How universal is this phenomenon? Does confinement drive invasive states in other cancer types besides melanoma?
- How reversible is it? If confinement is removed, do cells switch back, or is the invasive state “locked in”?
- Can HMGB2 or microtubule remodeling be safely targeted? If so, therapies could block the invasive transformation.
- How do mechanical cues interact with biochemical ones? Tumors experience multiple simultaneous stresses. Untangling the interplay could reveal new therapeutic strategies.
Answering these will require more work, but the direction is clear: cancer biology cannot be understood without considering physical forces.
Conclusion
This research adds a critical layer to our understanding of cancer. It shows that mechanical pressure from surrounding tissues can push melanoma cells into new states—less focused on rapid division but more invasive, mobile, and resistant to treatment.
The discovery of HMGB2’s central role, along with cytoskeletal adaptations that mimic neurons, highlights how deeply physical and genetic processes intertwine in cancer. For treatment, it suggests that targeting mechanosensitive pathways may be just as important as targeting genetic mutations or chemical signals.
By broadening the lens to include physical forces, researchers are uncovering vulnerabilities that might one day help stop cancer in its tracks.
Reference:
Hunter MV, Joshi E, Bowker S, Montal E, Ma Y, Kim YH, Yang Z, Tuffery L, Li Z, Rosiek E, Browning A, Moncada R, Yanai I, Byrne H, Monetti M, de Stanchina E, Hamard P-J, Koche RP, White RM. Mechanical confinement governs phenotypic plasticity in melanoma. Nature. 2025 Aug 27. doi: 10.1038/s41586-025-09445-6