Human Gene PARP14 Emerges as a Promising Target for Viral Diseases and Immune-Related Disorders
Researchers at the University of Kansas have uncovered new insights into the human gene PARP14, a protein that appears to play a surprising dual role in how the body responds to viral infections and inflammatory conditions. Their findings, published in the journal mBio, show that PARP14 both strengthens antiviral defenses and, in some cases, assists viral replication. This complex behavior positions the protein as a potential target for future treatments—not only for infections like COVID-19, herpes, or rabies-like viruses, but also for chronic immune-mediated conditions.
PARP14 is encoded in humans and all mammals, and the research team has shown clearly that this protein helps regulate interferon, one of the body’s most crucial innate immune molecules. Interferon acts as an early alarm system, triggering responses that slow down or block viral spread. The new study demonstrates that PARP14 supports this process, giving the immune system an additional layer of antiviral capability. According to the researchers, this is the first time PARP14 has been proven to exhibit antiviral activity across multiple classes of viruses.
The KU research group primarily studies coronaviruses, and this is where they initially observed PARP14’s antiviral effect. As coronaviruses attempt to evade immune defenses, PARP14 actively works to shut them down. The team describes this interaction as an arms race between host and virus. As the host evolves new antiviral tools, viruses develop countermeasures, each trying to gain the upper hand. PARP14 appears to be one of the host’s strong defensive tools.
Further collaborative work between researchers Anthony Fehr and David Davido expanded this discovery beyond coronaviruses. They found that PARP14 also restricts HSV-1 (herpes simplex virus), a widespread and persistent virus. This led to newly awarded grant funding to explore PARP14’s function across the broader herpesvirus family.
However, the story becomes more complicated with other viruses. PARP14 also shows proviral activity, meaning it actually supports the replication of certain viruses—specifically, rhabdoviruses such as the rabies virus. Instead of suppressing these viruses, PARP14 increases their ability to reproduce. This dual behavior offers both challenges and opportunities: while PARP14 can be bolstered to fight some viral infections, it may need to be inhibited to combat others.
Because of this complexity, PARP14 represents a unique therapeutic target. For infections like COVID-19 or herpes, drugs that enhance PARP14 activity might strengthen antiviral immunity. Conversely, for viruses that rely on PARP14 to replicate, inhibiting the protein could reduce viral load. The researchers point out that further investigation is needed to understand why PARP14 helps certain viruses while hindering others.
Beyond infections, PARP14 has implications for a much wider range of diseases. Since the protein is deeply involved in innate immunity, it could play a key role in inflammatory and autoimmune conditions. Overactive immune responses contribute to diseases such as diabetes and autoimmune disorders, and the researchers suggest that by inhibiting PARP14, it might be possible to dampen excessive inflammation. This opens the door to using PARP14 not just in antiviral strategies, but also in therapies aimed at conditions triggered or worsened by immune dysregulation.
The study has sparked new collaborations at KU, particularly with researchers studying the links between innate immunity and metabolic diseases. One such collaboration focuses on how PARP14 might influence inflammatory pathways associated with diabetes. Since PARP14 boosts interferon production, and interferon can trigger inflammatory cascades, uncovering this connection may lead to better understanding of how chronic inflammation contributes to metabolic disorders.
Understanding PARP14: A Deeper Look
Since this gene plays such a versatile role, it’s useful to understand what PARP14 actually is and how it functions on a molecular level.
What PARP14 Does Inside Cells
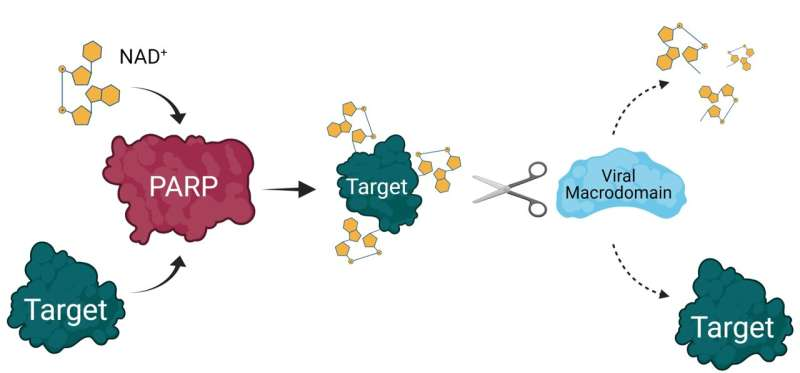
PARP14 belongs to the broader family of poly(ADP-ribose) polymerases or PARPs, proteins that add ADP-ribose molecules to substrates in a process called ADP-ribosylation. This modification affects how proteins behave, interact, and signal within the cell. Many PARPs are involved in DNA repair, inflammation, and immunity.
PARP14 has multiple functional regions, including:
- Macrodomains, which sense ADP-ribose modifications
- RNA-binding motifs
- A catalytic domain responsible for PARP14’s enzymatic activity
Through these features, PARP14 influences immune signaling pathways, particularly those surrounding interferons, which are activated early in viral infections.
The Host-Virus Arms Race
Many viruses, particularly coronaviruses, have evolved proteins that can remove ADP-ribose modifications, disabling the host’s antiviral tools. PARP14 is one of the host enzymes viruses aim to neutralize. This back-and-forth evolutionary pressure is evident in genetic analyses showing signs of positive selection on PARP14, hinting that it has been important in defending mammals against viruses across evolutionary history.
Why PARP14 Helps Some Viruses and Hurts Others
This paradox is one of the most interesting aspects of PARP14.
Some viruses rely on host immune pathways for aspects of their replication cycle. If PARP14 enhances parts of the immune response that those viruses exploit, it can unintentionally boost their replication. This appears to be the case with rhabdoviruses. On the other hand, viruses like coronaviruses and herpesviruses are hindered by the interferon signaling that PARP14 strengthens, giving PARP14 its antiviral profile there.
Future Therapeutic Possibilities
There are three major ways PARP14 might be targeted in medical treatments:
- Boosting PARP14 activity
- Potentially useful for viral infections where PARP14 helps restrict replication, such as COVID-19 or HSV-1.
- Inhibiting PARP14 activity
- Might help combat viruses like rhabdoviruses where PARP14 acts as a helper.
- Modulating immune and inflammatory pathways
- Could be relevant for chronic inflammatory diseases, autoimmune disorders, or inflammatory metabolic diseases such as certain forms of diabetes.
The key challenge is determining which direction—activation or inhibition—benefits the patient depending on the disease.
Why This Discovery Matters
PARP14 sits at a critical intersection of virology, immunology, and inflammation research. The fact that a single host protein:
- inhibits some viruses,
- supports other viruses, and
- regulates immune-driven inflammatory pathways
makes it an unusually valuable target for scientific exploration.
As researchers continue to study PARP14, we may see new classes of broad-spectrum antiviral drugs, new therapeutic approaches for chronic inflammation, and deeper insights into how the immune system balances protection with self-damage.
The recent findings mark an important step toward a more precise understanding of innate immunity and how we might leverage it to treat a wide variety of diseases.
Research Paper:
PARP14 is an interferon-induced host factor that promotes IFN production and affects the replication of multiple viruses
https://doi.org/10.1128/mbio.02299-25





