Medications Reshape the Gut Microbiome in Predictable and Long-Lasting Ways
Our gut is home to trillions of microbes that support digestion, immunity, metabolism, and even brain signaling. When this community becomes unbalanced, the effects can ripple throughout the entire body. While many people know that antibiotics can disrupt the gut microbiome, new research from Stanford University shows that a wide range of common medications can reshape it too — and in surprisingly predictable ways.
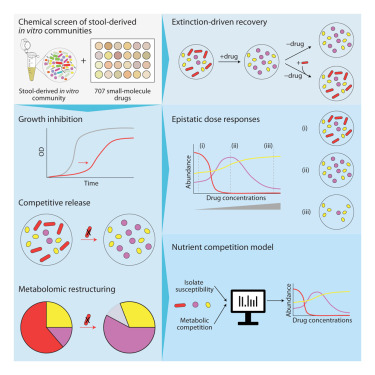
In a large-scale study published on November 17 in Cell, researchers examined how 707 clinically relevant drugs affect gut bacterial communities. Their findings reveal that drug-induced microbiome changes follow ecological rules, especially those involving nutrient competition. This means medications don’t just suppress or kill bacteria directly — they alter the availability of nutrients in the gut, and the microbes that best capitalize on those changes are the ones that survive or take over. This insight opens up possibilities for predicting, preventing, and potentially reversing the side effects that medications have on gut health.
How the Stanford Study Was Conducted
To understand how medications influence the microbiome, the researchers grew microbial communities from nine different human donors. These communities were exposed to more than 700 drugs, making this one of the most comprehensive drug–microbiome interaction studies conducted so far.
They systematically measured:
- Changes in the growth of individual bacterial species
- Shifts in overall community composition
- Alterations in the metabolome — the collection of small molecules microbes produce or consume
Out of the 707 drugs tested, 141 caused measurable changes in the microbial communities. Some medications wiped out entire species, and even short-term drug exposure sometimes resulted in persistent changes.
A key finding was that nutrient competition drives much of the community’s response. When a drug reduces certain bacterial populations, it alters which nutrients are abundant or scarce. This creates a new competitive landscape. Microbes that can exploit the remaining nutrients tend to dominate, while others decline. Therefore, the outcome of drug exposure depends on both drug sensitivity and resource competition.
Building Predictive Models of Microbial Change
Despite the complexity of the gut microbiome, the Stanford team built computational models that accurately predicted how microbial communities would reorganize under different drug treatments. The models incorporated two factors:
- How sensitive each bacterial species is to a specific drug
- How species compete with one another for available nutrients
This approach reframes drug effects from being microbe-targeted to being ecosystem-targeted. Instead of viewing a medication as acting on one organism, the study treats the gut microbiome as a dynamic ecological system.
The researchers found that community responses were consistent across different donors. Though each person’s microbiome is unique, the overarching patterns — which bacteria tend to rise or fall under certain conditions — followed similar rules. This consistency strengthens the potential for using such models in personalized medicine.
Long-Term Effects and Microbiome Recovery
Some of the drug-induced shifts persisted even after the drug was removed. Certain bacterial species did not rebound naturally once wiped out. However, the researchers tested whether reseeding — reintroducing the lost bacteria — could restore balance. In many cases, reseeding brought the community back to its earlier state, but not always. Some drugs pushed the community into new, stable configurations that did not revert on their own.
Interestingly, even though the drugs exerted strong pressures on the microbial community, the emergence of drug-resistant strains was rare. This contrasts with what typically happens when a single bacterium is exposed to a drug in isolation. The ecosystem context appears to reduce the selective advantage of resistance.
Implications for Medicine and Daily Life
This research suggests that medications commonly used for non-gut-related conditions can have unintended consequences for gut health. Certain drugs — such as those used for mood disorders, cardiovascular conditions, and gastric issues — have already been linked to microbiome disturbances in human population studies. These earlier findings line up with the new controlled laboratory evidence.
Understanding drug–microbiome interactions could influence:
- Drug design, encouraging pharmaceutical companies to consider microbiome safety
- Clinical prescribing, helping doctors choose medications that minimize gut disruption
- Personalized treatment, combining drugs with targeted diets or probiotics
- Microbiome recovery strategies, like reseeding or dietary adjustments
As the connection between gut bacteria and chronic conditions (including metabolic diseases, autoimmune disorders, and mental health issues) grows clearer, the importance of protecting microbial balance becomes more apparent.
What This Means for Future Research
The Stanford team plans to expand their work beyond small-molecule drugs. They are beginning similar tests with traditional herbal medicines, which have been used for thousands of years but remain poorly understood scientifically. Early evidence suggests some herbal compounds have strong effects on the microbiome.
There is also interest in studying how additional everyday factors — diet, water composition, exercise, and other environmental exposures — fit into the same ecological framework. The researchers aim to use ingestible sampling devices to examine microbiome changes in the small intestine in real time.
By focusing on how microbes compete for food, the researchers believe they can explain much of the “collateral damage” caused by medications. Knowing which bacteria are likely to survive or decline under certain conditions makes the gut’s response less mysterious and more predictable.
Extra Background: Why the Gut Microbiome Matters
The human gut microbiome acts almost like an organ of its own. It contributes to:
- Food digestion, especially of complex fibers
- Immune regulation, helping the body distinguish friend from foe
- Metabolite production, creating vitamins and short-chain fatty acids
- Brain communication, through the gut-brain axis
Disruptions to the microbiome — called dysbiosis — have been linked to inflammation, mental health shifts, obesity, allergies, and more. Medication-induced dysbiosis is becoming an area of increasing concern as researchers uncover its widespread effects.
The more we learn, the clearer it becomes that the microbiome responds to disturbances in ecological patterns. Drugs may change which microbes survive, but competition for food determines which microbes thrive. This interplay forms the foundation of microbiome resilience.
Extra Background: Nutrient Competition Explained
Nutrient competition refers to how microbes battle for the sugars, fibers, amino acids, and metabolites available in the gut. Each species is specialized to use certain nutrients. When medications suppress one group of bacteria, they free up specific nutrients, allowing other bacteria to expand rapidly.
For example:
- A drug may suppress bacteria that normally consume a certain carbohydrate.
- That carbohydrate becomes abundant.
- Bacteria capable of quickly metabolizing it multiply and take over.
This process helps explain why some drug effects are long-lasting: once a new microbial group establishes dominance, it can reshape the nutrient environment further, reinforcing its place in the community.
Conclusion
This new research provides one of the most comprehensive looks yet at how medications interact with the microbiome. By showing that changes follow predictable ecological rules, the study opens the door to better drug choices, personalized interventions, and improved strategies for protecting gut health. As science increasingly treats the microbiome as an ecosystem rather than a collection of isolated organisms, our understanding of its responses to medication will continue to deepen.
Research Paper:
Nutrient Competition Predicts Gut Microbiome Restructuring Under Drug Perturbations
https://doi.org/10.1016/j.cell.2025.10.038